Endometrial cancer
Active Ingredient: Dostarlimab
Indication for Dostarlimab
Dostarlimab is indicated as monotherapy for the treatment of adult patients with mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) recurrent or advanced endometrial cancer (EC) that has progressed on or following prior treatment with a platinum-containing regimen.
For this indication, competent medicine agencies globally authorize below treatments:
500 mg at weeks 1, 4, 7, 10 and thereafter 1.000 mg at week 13 and once every 6 weeks
For:
Dosage regimens
Intravenous, 500 milligrams dostarlimab, once every 3 weeks, 4 doses in total, over the duration of 13 weeks. Afterwards, intravenous, 1,000 milligrams dostarlimab, once every 6 weeks.
Detailed description
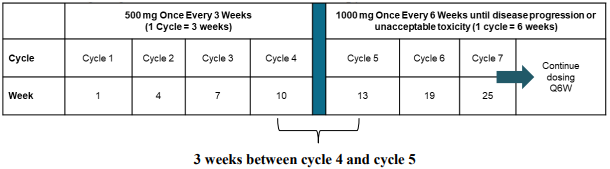
The recommended dose as monotherapy is 500 mg dostarlimab every 3 weeks for 4 cycles followed by 1000 mg every 6 weeks for all cycles thereafter.
The dosage regimen as monotherapy is presented in table 1.
Table 1. Dosage regimen for dostarlimab as monotherapy:
Administration of dostarlimab should continue according to the recommended schedule until disease progression or unacceptable toxicity.
Dose modifications
Dose reduction is not recommended. Dosing delay or discontinuation may be required based on individual safety and tolerability. Recommended modifications to manage adverse reactions are provided in Table 2.
Table 2. Recommended dose modifications for dostarlimab:
| Immune-related adverse reactions | Severity gradea | Dose modification,/b> |
| Colitis | 2 or 3 | Withhold dose. Restart dosing when toxicity resolves to grade 0 or 1. |
| 4 | Permanently discontinue. | |
| Hepatitis | Grade 2 with ASTb or ALTc > 3 and up to 5 × ULNd or total bilirubin > 1.5 and up to 3 × ULN | Withhold dose. Restart dosing when toxicity resolves to grade 0 or 1. |
| Grade ≥ 3 with AST or ALT > 5 × ULN or total bilirubin > 3 × ULN | Permanently discontinue (see exception below)e. | |
| Type 1 diabetes mellitus (T1DM) | 3 or 4 (hyperglycaemia) | Withhold dose. Restart dosing in appropriately managed, clinically and metabolically stable patients. |
| Hypophysitis or adrenal insufficiency | 2, 3 or 4 | Withhold dose. Restart dosing when toxicity resolves to grade 0 or 1. Permanently discontinue for recurrence or worsening while on adequate hormonal therapy. |
| Hypothyroidism or hyperthyroidism | 3 or 4 | Withhold dose. Restart dosing when toxicity resolves to grade 0 or 1. |
| Pneumonitis | 2 | Withhold dose. Restart dosing when toxicity resolves to grade 0 or 1. If grade 2 recurs, permanently discontinue. |
| 3 or 4 | Permanently discontinue. | |
| Nephritis | 2 | Withhold dose. Restart dosing when toxicity resolves to grade 0 or 1. |
| 3 or 4 | Permanently discontinue. | |
| Exfoliative dermatologic conditions (e.g. SJS, TEN, DRESS) | Suspected | Withhold dose for any grade. Restart dosing if not confirmed and when toxicity resolves to grade 0 or 1. |
| Confirmed | Permanently discontinue. | |
| Myocarditis | 2, 3 or 4 | Permanently discontinue. |
| Severe neurological toxicities (myasthenic syndrome/myasthenia gravis, Guillain-Barré syndrome, encephalitis, transverse myelitis) | 2, 3 or 4 | Permanently discontinue. |
| Other immune-related adverse reactions (including but not limited to myositis, sarcoidosis, autoimmune haemolytic anaemia, pancreatitis, iridocyclitis, uveitis, diabetic ketoacidosis, arthralgia, solid organ transplant rejection, graft- versus-host disease) | 3 | Withhold dose. Restart dosing when toxicity resolves to grade 0 or 1. |
| 4 | Permanently discontinue. | |
| Recurrence of immune-related adverse reactions after resolution to ≤ grade 1 (except for pneumonitis, see above) | 3 or 4 | Permanently discontinue. |
| Other adverse reactions | Severity gradea | Dose modification |
| Infusion-related reactions | 2 | Withhold dose. If resolved within 1 hour of stopping, may be restarted at 50% of the original infusion rate, or restart when symptoms resolve with pre-medication. If grade 2 recurs with adequate premedication, permanently discontinue. |
| 3 or 4 | Permanently discontinue. |
a Toxicity graded per National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
b AST = aspartate aminotransferase
c ALT = alanine aminotransferase
d ULN = upper limit of normal
e For patients with liver metastases who begin treatment with grade 2 increase of AST or ALT, if AST or ALT increases by ≥50% relative to baseline and lasts for at least 1 week, then treatment should be discontinued.
Dosage considerations
Dostarlimab is for intravenous infusion only. Dostarlimab should be administered by intravenous infusion using an intravenous infusion pump over 30 minutes.
Dostarlimab must not be administered as an intravenous push or bolus injection.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.