VITRAKVI Capsule / Oral solution Ref.[10108] Active ingredients: Larotrectinib
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
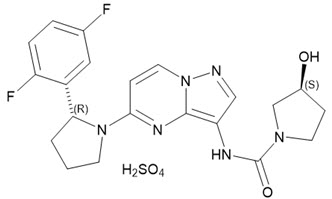
Larotrectinib is a kinase inhibitor. VITRAKVI (larotrectinib) capsules and oral solution are formulated using larotrectinib sulfate. The molecular formula for larotrectinib sulfate is C21H24F2N6O6S and the molecular weight is 526.51 g/mol for the sulfate salt and 428.44 g/mol for the free base.
The chemical name is (3S)N{5-[(2R)2(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-a]pyrimidin-3-yl}-3-hydroxy-1-pyrrolidinecarboxamide sulfate.
Larotrectinib sulfate has the following chemical structure:
Larotrectinib sulfate is an off-white to pinkish yellow solid that is not hygroscopic. The aqueous solubility of larotrectinib at 37°C is pH dependent (very soluble at pH 1.0 and freely soluble at pH 6.8, according to USP descriptive terms of solubility).
VITRAKVI (larotrectinib) capsules and oral solution are for oral use. Each capsule contains 25 mg or 100 mg larotrectinib (30.7 mg and 123 mg larotrectinib sulfate, respectively) in a hard gelatin capsule. The capsule is composed of gelatin, titanium dioxide, and edible ink.
The oral solution contains 20 mg/mL larotrectinib (24.6 mg/mL larotrectinib sulfate) and the following inactive ingredients: purified water, hydroxypropyl betadex, sucrose, glycerin, sorbitol, citric acid, sodium phosphate, sodium citrate dihydrate, propylene glycol and flavoring. Preserved with methylparaben and potassium sorbate.
| Dosage Forms and Strengths |
|---|
|
Capsules:
Oral Solution:
|
| How Supplied |
|---|
Capsules25 mg: Hard gelatin opaque white capsule size #2 with blue printing of “BAYER” cross and “25 mg” on the body of the capsule.
100 mg: Hard gelatin opaque white capsule size #0 with blue printing of “BAYER” cross and “100 mg” on the body of the capsule.
Oral Solution20 mg/mL: Clear yellow to orange solution.
|
Drugs
| Drug | Countries | |
|---|---|---|
| VITRAKVI | Austria, Brazil, Canada, Estonia, Finland, France, Hong Kong, Croatia, Ireland, Israel, Italy, Japan, Lithuania, Netherlands, Poland, Turkey, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.