EVISTA Film-coated tablet Ref.[10560] Active ingredients: Raloxifene
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Raloxifene is an estrogen agonist/antagonist, commonly referred to as a selective estrogen receptor modulator (SERM). The biological actions of raloxifene are largely mediated through binding to estrogen receptors. This binding results in activation of estrogenic pathways in some tissues (agonism) and blockade of estrogenic pathways in others (antagonism). The agonistic or antagonistic action of raloxifene depends on the extent of recruitment of coactivators and corepressors to estrogen receptor (ER) target gene promoters.
Raloxifene appears to act as an estrogen agonist in bone. It decreases bone resorption and bone turnover, increases bone mineral density (BMD) and decreases fracture incidence. Preclinical data demonstrate that raloxifene is an estrogen antagonist in uterine and breast tissues. These results are consistent with findings in clinical trials, which suggest that EVISTA lacks estrogen-like effects on the uterus and breast tissue.
12.2. Pharmacodynamics
Decreases in estrogen levels after oophorectomy or menopause lead to increases in bone resorption and accelerated bone loss. Bone is initially lost rapidly because the compensatory increase in bone formation is inadequate to offset resorptive losses. In addition to loss of estrogen, this imbalance between resorption and formation may be due to age-related impairment of osteoblasts or their precursors. In some women, these changes will eventually lead to decreased bone mass, osteoporosis, and increased risk for fractures, particularly of the spine, hip, and wrist. Vertebral fractures are the most common type of osteoporotic fracture in postmenopausal women.
In both the osteoporosis treatment and prevention trials, EVISTA therapy resulted in consistent, statistically significant suppression of bone resorption and bone formation, as reflected by changes in serum and urine markers of bone turnover (e.g., bone-specific alkaline phosphatase, osteocalcin, and collagen breakdown products). The suppression of bone turnover markers was evident by 3 months and persisted throughout the 36-month and 24-month observation periods.
In a 31-week, open-label, radiocalcium kinetics study, 33 early postmenopausal women were randomized to treatment with once-daily EVISTA 60 mg, cyclic estrogen/progestin (0.625 mg conjugated estrogens daily with 5 mg medroxyprogesterone acetate daily for the first 2 weeks of each month [hormone therapy]), or no treatment. Treatment with either EVISTA or hormone therapy was associated with reduced bone resorption and a positive shift in calcium balance (-82 mg Ca/day and +60 mg Ca/day, respectively, for EVISTA and -162 mg Ca/day and +91 mg Ca/day, respectively, for hormone therapy).
There were small decreases in serum total calcium, inorganic phosphate, total protein, and albumin, which were generally of lesser magnitude than decreases observed during estrogen or hormone therapy. Platelet count was also decreased slightly and was not different from estrogen therapy.
12.3. Pharmacokinetics
The disposition of raloxifene has been evaluated in more than 3000 postmenopausal women in selected raloxifene osteoporosis treatment and prevention clinical trials, using a population approach. Pharmacokinetic data also were obtained in conventional pharmacology studies in 292 postmenopausal women. Raloxifene exhibits high within-subject variability (approximately 30% coefficient of variation) of most pharmacokinetic parameters. Table 3 summarizes the pharmacokinetic parameters of raloxifene.
Absorption
Raloxifene is absorbed rapidly after oral administration. Approximately 60% of an oral dose is absorbed, but presystemic glucuronide conjugation is extensive. Absolute bioavailability of raloxifene is 2%. The time to reach average maximum plasma concentration and bioavailability are functions of systemic interconversion and enterohepatic cycling of raloxifene and its glucuronide metabolites.
Administration of raloxifene HCl with a standardized, high-fat meal increases the absorption of raloxifene (Cmax 28% and AUC 16%), but does not lead to clinically meaningful changes in systemic exposure. EVISTA can be administered without regard to meals.
Distribution
Following oral administration of single doses ranging from 30 to 150 mg of raloxifene HCl, the apparent volume of distribution is 2348 L/kg and is not dose dependent.
Raloxifene and the monoglucuronide conjugates are highly (95%) bound to plasma proteins. Raloxifene binds to both albumin and α1-acid glycoprotein, but not to sex-steroid binding globulin.
Metabolism
Biotransformation and disposition of raloxifene in humans have been determined following oral administration of 14C-labeled raloxifene. Raloxifene undergoes extensive first-pass metabolism to the glucuronide conjugates: raloxifene-4′-glucuronide, raloxifene-6-glucuronide, and raloxifene-6, 4′-diglucuronide. No other metabolites have been detected, providing strong evidence that raloxifene is not metabolized by cytochrome P450 pathways. Unconjugated raloxifene comprises less than 1% of the total radiolabeled material in plasma. The terminal log-linear portions of the plasma concentration curves for raloxifene and the glucuronides are generally parallel. This is consistent with interconversion of raloxifene and the glucuronide metabolites.
Following intravenous administration, raloxifene is cleared at a rate approximating hepatic blood flow. Apparent oral clearance is 44.1 L/kg•hr. Raloxifene and its glucuronide conjugates are interconverted by reversible systemic metabolism and enterohepatic cycling, thereby prolonging its plasma elimination half-life to 27.7 hours after oral dosing.
Results from single oral doses of raloxifene predict multiple-dose pharmacokinetics. Following chronic dosing, clearance ranges from 40 to 60 L/kg•hr. Increasing doses of raloxifene HCl (ranging from 30 to 150 mg) result in slightly less than a proportional increase in the area under the plasma time concentration curve (AUC).
Excretion
Raloxifene is primarily excreted in feces, and less than 0.2% is excreted unchanged in urine. Less than 6% of the raloxifene dose is eliminated in urine as glucuronide conjugates.
Table 3. Summary of Raloxifene Pharmacokinetic Parameters in the Healthy Postmenopausal Woman:
| Cmaxa,b (ng/mL)/(mg/kg) | t1/2 (hr)a | AUC0-∞a,b (ng•hr/mL)/(mg/kg) | CL/Fa (L/kg•hr) | V/Fa (L/kg) | |
|---|---|---|---|---|---|
| Single Dose Mean | 0.50 | 27.7 | 27.2 | 44.1 | 2348 |
| CVa (%) | 52 | 10.7 to 273c | 44 | 46 | 52 |
| Multiple Dose Mean | 1.36 | 32.5 | 24.2 | 47.4 | 2853 |
| CVa (%) | 37 | 15.8 to 86.6c | 36 | 41 | 56 |
a Abbreviations: Cmax = maximum plasma concentration, t1/2 = half-life, AUC = area under the curve, CL = clearance, V = volume of distribution, F = bioavailability, CV = coefficient of variation.
b Data normalized for dose in mg and body weight in kg.
c Range of observed half-life.
Special Populations
Pediatric
The pharmacokinetics of raloxifene has not been evaluated in a pediatric population [see Use in Specific Populations (8.4)].
Geriatric
No differences in raloxifene pharmacokinetics were detected with regard to age (range 42 to 84 years) [see Use in Specific Populations (8.5)].
Gender
Total extent of exposure and oral clearance, normalized for lean body weight, are not significantly different between age-matched female and male volunteers.
Race
Pharmacokinetic differences due to race have been studied in 1712 women, including 97.5% White, 1.0% Asian, 0.7% Hispanic, and 0.5% Black in the osteoporosis treatment trial and in 1053 women, including 93.5% White, 4.3% Hispanic, 1.2% Asian, and 0.5% Black in the osteoporosis prevention trials. There were no discernible differences in raloxifene plasma concentrations among these groups; however, the influence of race cannot be conclusively determined.
Renal Impairment
In the osteoporosis treatment and prevention trials, raloxifene concentrations in women with mild renal impairment are similar to women with normal creatinine clearance. When a single dose of 120 mg raloxifene HCl was administered to 10 renally impaired males [7 moderate impairment (CrCl = 31–50 mL/min); 3 severe impairment (CrCl ≤30 mL/min)] and to 10 healthy males (CrCl >80 mL/min), plasma raloxifene concentrations were 122% (AUC0-∞) higher in renally impaired patients than those of healthy volunteers. Raloxifene should be used with caution in patients with moderate or severe renal impairment [see Warnings and Precautions (5.8) and Use in Specific Populations (8.6)].
Hepatic Impairment
The disposition of raloxifene was compared in 9 patients with mild (Child-Pugh Class A) hepatic impairment (total bilirubin ranging from 0.6 to 2 mg/dL) to 8 subjects with normal hepatic function following a single dose of 60 mg raloxifene HCl. Apparent clearance of raloxifene was reduced 56% and the half-life of raloxifene was not altered in patients with mild hepatic impairment. Plasma raloxifene concentrations were approximately 150% higher than those in healthy volunteers and correlated with total bilirubin concentrations. The pharmacokinetics of raloxifene has not been studied in patients with moderate or severe hepatic impairment. Raloxifene should be used with caution in patients with hepatic impairment [see Warnings and Precautions (5.5) and Use in Specific Populations (8.7)].
Drug Interactions
Cholestyramine
Cholestyramine, an anion exchange resin, causes a 60% reduction in the absorption and enterohepatic cycling of raloxifene after a single dose. Although not specifically studied, it is anticipated that other anion exchange resins would have a similar effect [see Drug Interactions (7.1)].
Warfarin
In vitro, raloxifene did not interact with the binding of warfarin. The concomitant administration of EVISTA and warfarin, a coumarin derivative, has been assessed in a single-dose study. In this study, raloxifene had no effect on the pharmacokinetics of warfarin. However, a 10% decrease in prothrombin time was observed in the single-dose study. In the osteoporosis treatment trial, there were no clinically relevant effects of warfarin co-administration on plasma concentrations of raloxifene [see Drug Interactions (7.2)].
Other Highly Protein-Bound Drugs
In the osteoporosis treatment trial, there were no clinically relevant effects of co-administration of other highly protein-bound drugs (e.g., gemfibrozil) on plasma concentrations of raloxifene. In vitro, raloxifene did not interact with the binding of phenytoin, tamoxifen, or warfarin (see above) [see Drug Interactions (7.3)].
Ampicillin and Amoxicillin
Peak concentrations of raloxifene and the overall extent of absorption are reduced 28% and 14%, respectively, with co-administration of ampicillin. These reductions are consistent with decreased enterohepatic cycling associated with antibiotic reduction of enteric bacteria. However, the systemic exposure and the elimination rate of raloxifene were not affected. In the osteoporosis treatment trial, co-administration of amoxicillin had no discernible differences in plasma raloxifene concentrations [see Drug Interactions (7.5)].
Antacids
Concomitant administration of calcium carbonate or aluminum and magnesium hydroxide-containing antacids does not affect the systemic exposure of raloxifene [see Drug Interactions (7.5)].
Corticosteroids
The chronic administration of raloxifene in postmenopausal women has no effect on the pharmacokinetics of methylprednisolone given as a single oral dose [see Drug Interactions (7.5)].
Digoxin
Raloxifene has no effect on the pharmacokinetics of digoxin [see Drug Interactions (7.5)].
Cyclosporine
Concomitant administration of EVISTA with cyclosporine has not been studied.
Lipid-Lowering Agents
Concomitant administration of EVISTA with lipid-lowering agents has not been studied.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 21-month carcinogenicity study in mice, there was an increased incidence of ovarian tumors in female animals given 9 to 242 mg/kg, which included benign and malignant tumors of granulosa/theca cell origin and benign tumors of epithelial cell origin. Systemic exposure (AUC) of raloxifene in this group was 0.3 to 34 times that in postmenopausal women administered a 60 mg dose. There was also an increased incidence of testicular interstitial cell tumors and prostatic adenomas and adenocarcinomas in male mice given 41 or 210 mg/kg (4.7 or 24 times the AUC in humans) and prostatic leiomyoblastoma in male mice given 210 mg/kg.
In a 2-year carcinogenicity study in rats, an increased incidence in ovarian tumors of granulosa/theca cell origin was observed in female rats given 279 mg/kg (approximately 400 times the AUC in humans). The female rodents in these studies were treated during their reproductive lives when their ovaries were functional and responsive to hormonal stimulation.
Mutagenesis
Raloxifene HCl was not genotoxic in any of the following test systems: the Ames test for bacterial mutagenesis with and without metabolic activation, the unscheduled DNA synthesis assay in rat hepatocytes, the mouse lymphoma assay for mammalian cell mutation, the chromosomal aberration assay in Chinese hamster ovary cells, the in vivo sister chromatid exchange assay in Chinese hamsters, and the in vivo micronucleus test in mice.
Impairment of Fertility
When male and female rats were given daily doses ≥5 mg/kg (≥0.8 times the human dose based on surface area, mg/m²) prior to and during mating, no pregnancies occurred. In male rats, daily doses up to 100 mg/kg (16 times the human dose based on surface area, mg/m²) for at least 2 weeks did not affect sperm production or quality or reproductive performance. In female rats, at doses of 0.1 to 10 mg/kg/day (0.02 to 1.6 times the human dose based on surface area, mg/m²), raloxifene disrupted estrous cycles and inhibited ovulation. These effects of raloxifene were reversible. In another study in rats in which raloxifene was given during the preimplantation period at doses ≥0.1 mg/kg (≥0.02 times the human dose based on surface area, mg/m²), raloxifene delayed and disrupted embryo implantation, resulting in prolonged gestation and reduced litter size. The reproductive and developmental effects observed in animals are consistent with the estrogen receptor activity of raloxifene.
13.2. Animal Toxicology and/or Pharmacology
The skeletal effects of raloxifene treatment were assessed in ovariectomized rats and monkeys. In rats, raloxifene prevented increased bone resorption and bone loss after ovariectomy. There were positive effects of raloxifene on bone strength, but the effects varied with time. Cynomolgus monkeys were treated with raloxifene or conjugated estrogens for 2 years. In terms of bone cycles, this is equivalent to approximately 6 years in humans. Raloxifene and estrogen suppressed bone turnover and increased BMD in the lumbar spine and in the central cancellous bone of the proximal tibia. In this animal model, there was a positive correlation between vertebral compressive breaking force and BMD of the lumbar spine.
Histologic examination of bone from rats and monkeys treated with raloxifene showed no evidence of woven bone, marrow fibrosis, or mineralization defects.
These results are consistent with data from human studies of radiocalcium kinetics and markers of bone metabolism, and are consistent with the action of EVISTA as a skeletal antiresorptive agent.
14. Clinical Studies
14.1 Treatment of Postmenopausal Osteoporosis
Effect on Fracture Incidence
The effects of EVISTA on fracture incidence and BMD in postmenopausal women with osteoporosis were examined at 3 years in a large randomized, placebo-controlled, double-blind, multinational osteoporosis treatment trial (MORE). All vertebral fractures were diagnosed radiographically; some of these fractures also were associated with symptoms (i.e., clinical fractures). The study population consisted of 7705 postmenopausal women with osteoporosis as defined by: a) low BMD (vertebral or hip BMD at least 2.5 standard deviations below the mean value for healthy young women) without baseline vertebral fractures or b) one or more baseline vertebral fractures. Women enrolled in this study had a median age of 67 years (range 31 to 80) and a median time since menopause of 19 years.
Effect on Bone Mineral Density
EVISTA, 60 mg administered once daily, increased spine and hip BMD by 2 to 3%. EVISTA decreased the incidence of the first vertebral fracture from 4.3% for placebo to 1.9% for EVISTA (relative risk reduction = 55%) and subsequent vertebral fractures from 20.2% for placebo to 14.1% for EVISTA (relative risk reduction = 30%) (see Table 4). All women in the study received calcium (500 mg/day) and vitamin D (400 to 600 IU/day). EVISTA reduced the incidence of vertebral fractures whether or not patients had a vertebral fracture upon study entry. The decrease in incidence of vertebral fracture was greater than could be accounted for by increase in BMD alone.
Table 4. Effect of EVISTA on Risk of Vertebral Fractures:
| Number of Patients | Absolute Risk Reduction (ARR) | Relative Risk Reduction (95% CI) | ||
|---|---|---|---|---|
| EVISTA | Placebo | |||
| Fractures diagnosed radiographically | ||||
| Patients with no baseline fracturea | n=1401 | n=1457 | ||
| Number (%) of patients with ≥1 new vertebral fracture | 27 (1.9%) | 62 (4.3%) | 2.4% | 55% (29%, 71%) |
| Patients with ≥1 baseline fracturea | n=858 | n=835 | ||
| Number (%) of patients with ≥1 new vertebral fracture | 121 (14.1%) | 169 (20.2%) | 6.1% | 30% (14%, 44%) |
| Symptomatic vertebral fractures | ||||
| All randomized patients | n=2557 | n=2576 | ||
| Number (%) of patients with ≥1 new clinical (painful) vertebral fracture | 47 (1.8%) | 81 (3.1%) | 1.3% | 41% (17%, 59%) |
a Includes all patients with baseline and at least one follow-up radiograph.
The mean percentage change in BMD from baseline for EVISTA was statistically significantly greater than for placebo at each skeletal site (see Table 5).
Table 5. EVISTA- (60 mg Once Daily) Related Increases in BMDa for the Osteoporosis Treatment Study Expressed as Mean Percentage Increase vs. Placebob,c:
| Site | Time | ||
|---|---|---|---|
| 12 Months % | 24 Months % | 36 Months % | |
| Lumbar Spine | 2.0 | 2.6 | 2.6 |
| Femoral Neck | 1.3 | 1.9 | 2.1 |
| Ultradistal Radius | NDd | 2.2 | NDd |
| Distal Radius | NDd | 0.9 | NDd |
| Total Body | NDd | 1.1 | NDd |
a Note: all BMD increases were significant (p<0.001).
b Intent-to-treat analysis; last observation carried forward.
c All patients received calcium and vitamin D.
d ND = not done (total body and radius BMD were measured only at 24 months).
Discontinuation from the study was required when excessive bone loss or multiple incident vertebral fractures occurred. Such discontinuation was statistically significantly more frequent in the placebo group (3.7%) than in the EVISTA group (1.1%).
Bone Histology
Bone biopsies for qualitative and quantitative histomorphometry were obtained at baseline and after 2 years of treatment. There were 56 paired biopsies evaluable for all indices. In EVISTA-treated patients, there were statistically significant decreases in bone formation rate per tissue volume, consistent with a reduction in bone turnover. Normal bone quality was maintained; specifically, there was no evidence of osteomalacia, marrow fibrosis, cellular toxicity, or woven bone after 2 years of treatment.
Effect on Endometrium
Endometrial thickness was evaluated annually in a subset of the study population (1781 patients) for 3 years. Placebo-treated women had a 0.27 mm mean decrease from baseline in endometrial thickness over 3 years, whereas the EVISTA-treated women had a 0.06 mm mean increase. Patients in the osteoporosis treatment study were not screened at baseline or excluded for pre-existing endometrial or uterine disease. This study was not specifically designed to detect endometrial polyps. Over the 36 months of the study, clinically or histologically benign endometrial polyps were reported in 17 of 1999 placebo-treated women, 37 of 1948 EVISTA-treated women, and in 31 of 2010 women treated with raloxifene HCl 120 mg/day. There was no difference between EVISTA- and placebo-treated women in the incidences of endometrial carcinoma, vaginal bleeding, or vaginal discharge.
14.2 Prevention of Postmenopausal Osteoporosis
The effects of EVISTA on BMD in postmenopausal women were examined in three randomized, placebo-controlled, double-blind osteoporosis prevention trials: (1) a North American trial enrolled 544 women; (2) a European trial, 601 women; and (3) an international trial, 619 women who had undergone hysterectomy. In these trials, all women received calcium supplementation (400 to 600 mg/day). Women enrolled in these trials had a median age of 54 years and a median time since menopause of 5 years (less than 1 year up to 15 years postmenopause). The majority of the women were White (93.5%). Women were included if they had spine BMD between 2.5 standard deviations below and 2 standard deviations above the mean value for healthy young women. The mean T scores (number of standard deviations above or below the mean in healthy young women) for the three trials ranged from -1.01 to -0.74 for spine BMD and included women both with normal and low BMD. EVISTA, 60 mg administered once daily, produced increases in bone mass versus calcium supplementation alone, as reflected by dual-energy x-ray absorptiometric (DXA) measurements of hip, spine, and total body BMD.
Effect on Bone Mineral Density
Compared with placebo, the increases in BMD for each of the three studies were statistically significant at 12 months and were maintained at 24 months (see Table 6). The placebo groups lost approximately 1% of BMD over 24 months.
Table 6. EVISTA- (60 mg Once Daily) Related Increases in BMDa for the Three Osteoporosis Prevention Studies Expressed as Mean Percentage Increase vs. Placebob at 24 Monthsc:
| Site | Study | ||
|---|---|---|---|
| NAd % | EUd % | INTd,e % | |
| Total Hip | 2.0 | 2.4 | 1.3 |
| Femoral Neck | 2.1 | 2.5 | 1.6 |
| Trochanter | 2.2 | 2.7 | 1.3 |
| Intertrochanter | 2.3 | 2.4 | 1.3 |
| Lumbar Spine | 2.0 | 2.4 | 1.8 |
a Note: all BMD increases were significant (p≤0.001).
b All patients received calcium.
c Intent-to-treat analysis; last observation carried forward.
d Abbreviations: NA = North American, EU = European, INT = International.
e All women in the study had previously undergone hysterectomy.
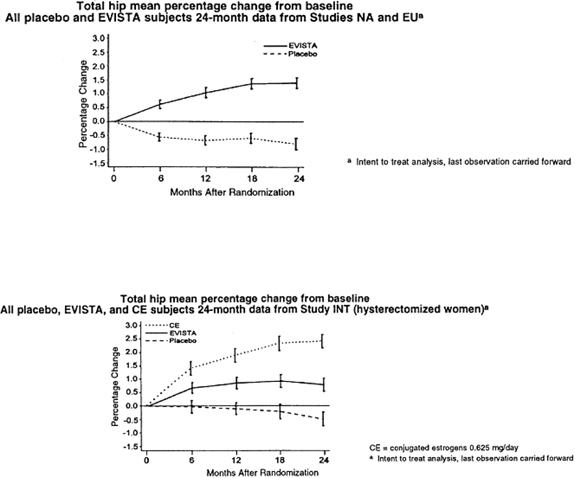
EVISTA also increased BMD compared with placebo in the total body by 1.3% to 2.0% and in Ward’s Triangle (hip) by 3.1% to 4.0%. The effects of EVISTA on forearm BMD were inconsistent between studies. In Study EU, EVISTA prevented bone loss at the ultradistal radius, whereas in Study NA, it did not (see Figure 1).
Figure 1. Total hip bone mineral density mean percentage change from bas eline:
Effect on Endometrium
In placebo-controlled osteoporosis prevention trials, endometrial thickness was evaluated every 6 months (for 24 months) by transvaginal ultrasonography (TVU). A total of 2978 TVU measurements were collected from 831 women in all dose groups. Placebo-treated women had a 0.04 mm mean increase from baseline in endometrial thickness over 2 years, whereas the EVISTA-treated women had a 0.09 mm mean increase. Endometrial thickness measurements in raloxifene-treated women were indistinguishable from placebo. There were no differences between the raloxifene and placebo groups with respect to the incidence of reported vaginal bleeding.
14.3 Reduction in Risk of Invasive Breast Cancer in Postmenopausal Women with Osteoporosis
MORE Trial
The effect of EVISTA on the incidence of breast cancer was assessed as a secondary safety endpoint in a randomized, placebo-controlled, double-blind, multinational osteoporosis treatment trial in postmenopausal women [see Clinical Studies (14.1)]. After 4 years, EVISTA, 60 mg administered once daily, reduced the incidence of all breast cancers by 62%, compared with placebo (HR 0.38, 95% CI 0.22-0.67). EVISTA reduced the incidence of invasive breast cancer by 71%, compared with placebo (ARR 3.1 per 1000 women-years); this was primarily due to an 80% reduction in the incidence of ER-positive invasive breast cancer in the EVISTA group compared with placebo. Table 7 presents efficacy and selected safety outcomes.
CORE Trial
The effect of EVISTA on the incidence of invasive breast cancer was evaluated for 4 additional years in a follow-up study conducted in a subset of postmenopausal women originally enrolled in the MORE osteoporosis treatment trial. Women were not re-randomized; the treatment assignment from the osteoporosis treatment trial was carried forward to this study. EVISTA, 60 mg administered once daily, reduced the incidence of invasive breast cancer by 56%, compared with placebo (ARR 3.0 per 1000 women-years); this was primarily due to a 63% reduction in the incidence of ER-positive invasive breast cancer in the EVISTA group compared with placebo. There was no reduction in the incidence of ER-negative breast cancer. In the osteoporosis treatment trial and the follow-up study, there was no difference in incidence of noninvasive breast cancer between the EVISTA and placebo groups. Table 7 presents efficacy and selected safety outcomes.
In a subset of postmenopausal women followed for up to 8 years from randomization in MORE to the end of CORE, EVISTA, 60 mg administered once daily, reduced the incidence of invasive breast cancer by 60% in women assigned EVISTA (N=1355) compared with placebo (N=1286) (HR 0.40, 95% CI 0.21, 0.77; ARR 1.95 per 1000 women-years); this was primarily due to a 65% reduction in the incidence of ER-positive invasive breast cancer in the EVISTA group compared with placebo.
Table 7. EVISTA (60 mg Once Daily) vs. Placebo on Outcomes in Postmenopausal Women with Osteoporosis:
| Outcomes | MORE 4 years | COREa 4 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (N=2576) | EVISTA (N=2557) | HR (95% CI)b | Placebo (N=1286) | EVISTA (N=2725) | HR (95% CI)b | |||||
| n | IRb | n | IRb | n | IRb | n | IRb | |||
| Invasivec breast cancer | 38 | 4.36 | 11 | 1.26 | 0.29 (0.15, 0.56)d | 20 | 5.41 | 19 | 2.43 | 0.44 (0.24, 0.83)d |
| ERb,c positive | 29 | 3.33 | 6 | 0.69 | 0.20 (0.08, 0.49) | 15 | 4.05 | 12 | 1.54 | 0.37 (0.17, 0.79) |
| ERb,c negative | 4 | 0.46 | 5 | 0.57 | 1.23 (0.33, 4.60) | 3 | 0.81 | 6 | 0.77 | 0.95 (0.24, 3.79) |
| ERb,c unknown | 5 | 0.57 | 0 | 0.00 | N/Ab | 2 | 0.54 | 1 | 0.13 | N/Ab |
| Noninvasivec,e breast cancer | 5 | 0.57 | 3 | 0.34 | 0.59 (0.14, 2.47) | 2 | 0.54 | 5 | 0.64 | 1.18 (0.23, 6.07) |
| Clinical vertebral fractures | 107 | 12.27 | 62 | 7.08 | 0.57 (0.42, 0.78) | N/Ab | N/Ab | N/Ab | N/Ab | N/Ab |
| Death | 36 | 4.13 | 23 | 2.63 | 0.63 (0.38, 1.07) | 29 | 7.76 | 47 | 5.99 | 0.77 (0.49, 1.23) |
| Death due to stroke | 6 | 0.69 | 3 | 0.34 | 0.49 (0.12, 1.98) | 1 | 0.27 | 6 | 0.76 | 2.87 (0.35, 23.80) |
| Stroke | 56 | 6.42 | 43 | 4.91 | 0.76 (0.51, 1.14) | 14 | 3.75 | 49 | 6.24 | 1.67 (0.92, 3.03) |
| Deep vein thrombosis | 8 | 0.92 | 20 | 2.28 | 2.50 (1.10, 5.68) | 4 | 1.07 | 17 | 2.17 | 2.03 (0.68, 6.03) |
| Pulmonary embolism | 4 | 0.46 | 11 | 1.26 | 2.76 (0.88, 8.67) | 0 | 0.00 | 9 | 1.15 | N/Ab |
| Endometrial and uterine cancerf | 5 | 0.74 | 5 | 0.74 | 1.01 (0.29, 3.49) | 3 | 1.02 | 4 | 0.65 | 0.64 (0.14, 2.85) |
| Ovarian cancer | 6 | 0.69 | 3 | 0.34 | 0.49 (0.12, 1.95) | 2 | 0.54 | 2 | 0.25 | 0.47 (0.07, 3.36) |
| Hot flashes | 151 | 17.31 | 237 | 27.06 | 1.61 (1.31, 1.97) | 11 | 2.94 | 26 | 3.31 | 1.12 (0.55, 2.27) |
| Peripheral edema | 134 | 15.36 | 164 | 18.73 | 1.23 (0.98, 1.54) | 30 | 8.03 | 61 | 7.77 | 0.96 (0.62, 1.49) |
| Cholelithiasis | 45 | 5.16 | 53 | 6.05 | 1.18 (0.79, 1.75) | 12 | 3.21 | 35 | 4.46 | 1.39 (0.72, 2.67) |
a CORE was a follow-up study conducted in a subset of 4011 postmenopausal women who originally enrolled in MORE. Women were not re-randomized; the treatment assignment from MORE was carried forward to this study. At CORE enrollment, the EVISTA group included 2725 total patients with 1355 patients who were originally assigned to raloxifene HCl 60 mg once daily and 1370 patients who were originally assigned to raloxifene HCl 120 mg at MORE randomization.
b Abbreviations: CI = confidence interval; ER = estrogen receptor; HR = hazard ratio; IR = annual incidence rate per 1000 women; N/A = not applicable.
c Included 1274 patients in placebo and 2716 patients in EVISTA who were not diagnosed with breast cancer prior to CORE enrollment.
d p<0.05, obtained from the log-rank test, and not adjusted for multiple comparisons in MORE.
e All cases were ductal carcinoma in situ.
f Only patients with an intact uterus were included (MORE: placebo = 1999, EVISTA = 1950; CORE: placebo = 1008, EVISTA = 2138).
RUTH Trial
The effect of EVISTA on the incidence of invasive breast cancer was assessed in a randomized, placebo-controlled, double-blind, multinational study in 10,101 postmenopausal women at increased risk of coronary events. Women in this study had a median age of 67.6 years (range 55-92) and were followed for a median of 5.6 years (range 0.01-7.1). Eighty-four percent were White, 9.8% of women reported a first-degree relative with a history of breast cancer, and 41.4% of the women had a 5-year predicted risk of invasive breast cancer ≥1.66%, based on the modified Gail model.
EVISTA, 60 mg administered once daily, reduced the incidence of invasive breast cancer by 44% compared with placebo [absolute risk reduction (ARR) 1.2 per 1000 women-years]; this was primarily due to a 55% reduction in estrogen receptor (ER)-positive invasive breast cancer in the EVISTA group compared with placebo (ARR 1.2 per 1000 women-years). There was no reduction in ER-negative invasive breast cancer. Table 8 presents efficacy and selected safety outcomes.
Table 8. EVISTA (60 mg Once Daily) vs. Placebo on Outcomes in Postmenopausal Women at Increased Risk for Major Coronary Events:
| Outcomes | Placeboa (N=5057) | EVISTAa (N=5044) | HR (95% CI)b | ||
|---|---|---|---|---|---|
| n | IRb | n | IRb | ||
| Invasive breast cancer | 70 | 2.66 | 40 | 1.50 | 0.56 (0.38, 0.83)c |
| ERb positive | 55 | 2.09 | 25 | 0.94 | 0.45 (0.28, 0.72) |
| ERb negative | 9 | 0.34 | 13 | 0.49 | 1.44 (0.61, 3.36) |
| ERb unknown | 6 | 0.23 | 2 | 0.07 | 0.33 (0.07, 1.63) |
| Noninvasived breast cancer | 5 | 0.19 | 11 | 0.41 | 2.17 (0.75, 6.24) |
| Clinical vertebral fractures | 97 | 3.70 | 64 | 2.40 | 0.65 (0.47, 0.89) |
| Death | 595 | 22.45 | 554 | 20.68 | 0.92 (0.82, 1.03) |
| Death due to stroke | 39 | 1.47 | 59 | 2.20 | 1.49 (1.00, 2.24) |
| Stroke | 224 | 8.60 | 249 | 9.46 | 1.10 (0.92, 1.32) |
| Deep vein thrombosis | 47 | 1.78 | 65 | 2.44 | 1.37 (0.94, 1.99) |
| Pulmonary embolism | 24 | 0.91 | 36 | 1.35 | 1.49 (0.89, 2.49) |
| Endometrial and uterine cancere | 17 | 0.83 | 21 | 1.01 | 1.21 (0.64-2.30) |
| Ovarian cancerf | 10 | 0.41 | 17 | 0.70 | 1.69 (0.78, 3.70) |
| Hot flashes | 241 | 9.09 | 397 | 14.82 | 1.68 (1.43, 1.97) |
| Peripheral edema | 583 | 22.00 | 706 | 26.36 | 1.22 (1.09, 1.36) |
| Cholelithiasisg | 131 | 6.20 | 168 | 7.83 | 1.26 (1.01, 1.59) |
a Note: There were a total of 76 breast cancer cases in the placebo group and 52 in the EVISTA group. For two cases, one in each treatment group, invasive status was unknown.
b Abbreviations: CI = confidence interval; ER = estrogen receptor; HR = hazard ratio; IR = annual incidence rate per 1000 women.
c p<0.05, obtained from the log-rank test, after adjusting for the co-primary endpoint of major coronary events.
d All cases were ductal carcinoma in situ.
e Only patients with an intact uterus were included (placebo = 3882, EVISTA = 3900).
f Only patients with at least one ovary were included (placebo = 4606, EVISTA = 4559).
g Only patients with an intact gallbladder at baseline were included (placebo = 4111, EVISTA = 4144).
The effect of EVISTA in reducing the incidence of invasive breast cancer was consistent among women above or below age 65 or with a 5-year predicted invasive breast cancer risk, based on the modified Gail model, <1.66%, or ≥1.66%.
14.4 Reduction in Risk of Invasive Breast Cancer in Postmenopausal Women at High Risk of Invasive Breast Cancer
STAR Trial
The effects of EVISTA 60 mg/day versus tamoxifen 20 mg/day over 5 years on reducing the incidence of invasive breast cancer were assessed in 19,747 postmenopausal women in a randomized, double-blind trial conducted in North America by the National Surgical Adjuvant Breast and Bowel Project and sponsored by the National Cancer Institute. Women in this study had a mean age of 58.5 years (range 35-83), a mean 5-year predicted invasive breast cancer risk of 4.03% (range 1.66-23.61%), and 9.1% had a history of lobular carcinoma in situ (LCIS). More than 93% of participants were White. As of 31 December 2005, the median time of follow-up was 4.3 years (range 0.07-6.50 years).
EVISTA was not superior to tamoxifen in reducing the incidence of invasive breast cancer. The observed incidence rates of invasive breast cancer were EVISTA 4.4 and tamoxifen 4.3 per 1000 women per year. The results from a noninferiority analysis are consistent with EVISTA potentially losing up to 35% of the tamoxifen effect on reduction of invasive breast cancer. The effect of each treatment on invasive breast cancer was consistent when women were compared by baseline age, history of LCIS, history of atypical hyperplasia, 5-year predicted risk of breast cancer by the modified Gail model, or the number of relatives with a history of breast cancer. Fewer noninvasive breast cancers occurred in the tamoxifen group compared to the EVISTA group. Table 9 presents efficacy and selected safety outcomes.
Table 9. EVISTA (60 mg Once Daily) vs. Tamoxifen (20 mg Once Daily) on Outcomes in Postmenopausal Women at Increased Risk for Invasive Breast Cancer:
| Outcomes | EVISTA (N=9751) | Tamoxifen (N=9736) | RR (95% CI)a | ||
|---|---|---|---|---|---|
| n | IRa | n | IRa | ||
| Invasive breast cancer | 173 | 4.40 | 168 | 4.30 | 1.02 (0.82, 1.27) |
| ERa positive | 115 | 2.93 | 120 | 3.07 | 0.95 (0.73, 1.24) |
| ERa negative | 52 | 1.32 | 46 | 1.18 | 1.12 (0.74, 1.71) |
| ERa unknown | 6 | 0.15 | 2 | 0.05 | 2.98 (0.53, 30.21) |
| Noninvasive breast cancerb | 83 | 2.12 | 60 | 1.54 | 1.38 (0.98, 1.95) |
| DCISa | 47 | 1.20 | 32 | 0.82 | 1.46 (0.91, 2.37) |
| LCISa | 29 | 0.74 | 23 | 0.59 | 1.26 (0.70, 2.27) |
| Uterine cancerc | 23 | 1.21 | 37 | 1.99 | 0.61 (0.34, 1.05) |
| Endometrial hyperplasiac | 17 | 0.90 | 100 | 5.42 | 0.17 (0.09, 0.28) |
| Hysterectomyc | 92 | 4.84 | 246 | 13.25 | 0.37 (0.28, 0.47) |
| Ovarian cancerd | 18 | 0.66 | 14 | 0.52 | 1.27 (0.60, 2.76) |
| Ischemic heart diseasee | 138 | 3.50 | 125 | 3.19 | 1.10 (0.86, 1.41) |
| Stroke | 54 | 1.36 | 56 | 1.42 | 0.96 (0.65, 1.42) |
| Deep vein thrombosis | 67 | 1.69 | 92 | 2.35 | 0.72 (0.52, 1.00) |
| Pulmonary embolism | 38 | 0.96 | 58 | 1.47 | 0.65 (0.42, 1.00) |
| Clinical vertebral fractures | 58 | 1.46 | 58 | 1.47 | 0.99 (0.68, 1.46) |
| Cataractsf | 343 | 10.34 | 435 | 13.19 | 0.78 (0.68, 0.91) |
| Cataract surgeryf | 240 | 7.17 | 295 | 8.85 | 0.81 (0.68, 0.96) |
| Death | 104 | 2.62 | 109 | 2.76 | 0.95 (0.72, 1.25) |
| Edemag | 741 | 18.66 | 664 | 16.83 | 1.11 (1.00, 1.23) |
| Hot flashes | 6748 | 169.91 | 7170 | 181.71 | 0.94 (0.90, 0.97) |
a Abbreviations: CI = confidence interval; DCIS = ductal carcinoma in situ; ER = estrogen receptor; IR = annual incidence rate per 1000 women; LCIS = lobular carcinoma in situ; RR = risk ratio for women in the EVISTA group compared with those in the tamoxifen group.
b Of the 60 noninvasive breast cases in the tamoxifen group, 5 were mixed types. Of the 83 noninvasive breast cancers in the raloxifene group, 7 were mixed types.
c Only patients with an intact uterus at baseline were included (tamoxifen = 4739, EVISTA = 4715).
d Only patients with at least one intact ovary at baseline were included (tamoxifen = 6813, EVISTA = 6787).
e Defined as myocardial infarction, severe angina, or acute ischemic syndromes.
f Only patients who were free of cataracts at baseline were included (tamoxifen = 8342, EVISTA = 8333).
g Peripheral edema events are included in the term edema.
14.5 Effects on Cardiovascular Disease
In a randomized, placebo-controlled, double-blind, multinational clinical trial (RUTH) of 10,101 postmenopausal women with documented coronary heart disease or at increased risk for coronary events, no cardiovascular benefit was demonstrated after treatment with EVISTA 60 mg once daily for a median follow-up of 5.6 years. No significant increase or decrease was observed for coronary events (death from coronary causes, nonfatal myocardial infarction, or hospitalization for an acute coronary syndrome). An increased risk of death due to stroke after treatment with EVISTA was observed: 59 (1.2%) EVISTA-treated women died due to a stroke compared to 39 (0.8%) placebo-treated women (2.2 versus 1.5 per 1000 women-years; hazard ratio 1.49; 95% confidence interval, 1.00-2.24; p=0.0499). The incidence of stroke did not differ significantly between treatment groups (249 with EVISTA [4.9%] versus 224 with placebo [4.4%]; hazard ratio 1.10; 95% confidence interval 0.92-1.32; p=0.30; 9.5 versus 8.6 per 1000 women-years) [see Warnings and Precautions (5.2, 5.3)].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.