NASONEX Nasal spray Ref.[10599] Active ingredients: Mometasone
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
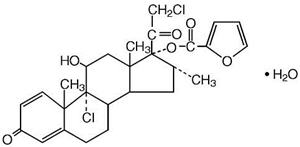
Mometasone furoate monohydrate, the active component of NASONEX Nasal Spray, 50 mcg, is an anti-inflammatory corticosteroid having the chemical name, 9,21-Dichloro-11ß,17-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione17-(2 furoate) monohydrate, and the following chemical structure:
Mometasone furoate monohydrate is a white powder, with an empirical formula of C27H30Cl2O6•H2O, and a molecular weight of 539.45. It is practically insoluble in water; slightly soluble in methanol, ethanol, and isopropanol; soluble in acetone and chloroform; and freely soluble in tetrahydrofuran. Its partition coefficient between octanol and water is greater than 5000.
NASONEX is a metered-dose, manual pump spray. After initial priming (10 actuations), each actuation of the pump delivers a metered spray containing 100 mcg or 100 microliter of aqueous suspension of mometasone furoate monohydrate equivalent to 50 mcg (0.05% w/w) mometasone furoate calculated on the anhydrous basis; in an aqueous medium containing glycerin, microcrystalline cellulose and carboxymethylcellulose sodium, sodium citrate, citric acid, benzalkonium chloride, and polysorbate 80. The pH is between 4.3 and 4.9.
| Dosage Forms and Strengths |
|---|
|
Nasal spray: 50 mcg, metered-dose, manual pump spray. After initial priming (10 actuations), each actuation of the pump delivers a metered spray containing 50 mcg of mometasone furoate. |
| How Supplied |
|---|
|
NASONEX Nasal Spray:
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA Manufactured by: MSD International GmbH (Singapore Branch), Singapore 638414, Singapore |
Drugs
| Drug | Countries | |
|---|---|---|
| NASONEX | Austria, Brazil, Canada, Germany, Ecuador, Estonia, Spain, Finland, France, Hong Kong, Croatia, Ireland, Japan, Lithuania, Malta, Netherlands, New Zealand, Poland, Romania, Singapore, Tunisia, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.