MARGENZA Concentrate for solution for injection Ref.[108707] Active ingredients: Margetuximab
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
Margetuximab-cmkb binds to the extracellular domain of the human epidermal growth factor receptor 2 protein (HER2). Upon binding to HER2-expressing tumor cells, margetuximab-cmkb inhibits tumor cell proliferation, reduces shedding of the HER2 extracellular domain and mediates antibody-dependent cellular cytotoxicity (ADCC).
In vitro, the modified Fc region of margetuximab-cmkb increases binding to activating Fc receptor FCGR3A (CD16A) and decreases binding to inhibitory Fc receptor FCGR2B (CD32B). These changes lead to greater in vitro ADCC and NK cell activation.
12.2. Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of margetuximab-cmkb have not been fully characterized.
12.3. Pharmacokinetics
Following the approved recommended dosage, the steady-state geometric mean (CV) Cmax of margetuximab-cmkb is 466 (20) µg/mL and AUC0-21d is 4120 (21%) µg.day/mL in patients with HER2-positive relapsed or refractory advanced breast cancer. Margetuximab-cmkb undergoes both linear and nonlinear elimination. After a single dose, margetuximab-cmkb Cmax and AUC0-21d increase in an approximately dose proportional manner from 10 to 18 mg/kg (0.67 to 1.2 times the approved recommended dose). At the approved recommended dosage, time to steady-state was 2 months, and accumulation ratio was 1.65 based on AUC0-21d. No clinically significant differences in margetuximab-cmkb exposure were observed when infusion time was reduced from 120 minutes to 30 minutes.
Distribution
Margetuximab-cmkb geometric mean (CV) steady-state volume of distribution is 5.47 L (22).
Elimination
The geometric mean (CV) terminal half-life of margetuximab-cmkb is 19.2 days (28) and clearance is 0.22 L/day (24%). Four months after margetuximab-cmkb discontinuation, concentrations decrease to approximately 3% of the steady-state trough serum concentration.
Metabolism
Margetuximab-cmkb is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in margetuximab-cmkb PK were observed based on age (29 to 83 years), sex, race (Caucasian, Black, Asian), mild to moderate (CLcr 30 to 89 mL/min estimated using the Cockcroft-Gault equation) renal impairment, mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN, or total bilirubin 1 to 1.5 ULN and any AST), HER2 expression level (0 to 3 by IHC), tumor burden (2–317 mm), ECOG score (0 to 2), albumin (24 to 50 g/L), FCGR3A (CD16A), FCGR2A (CD32A) and FCGR2B (CD32B) genotype, number of metastatic sites (≤2 or >2), number of prior therapy lines (≤2 or >2) or concurrent chemotherapies (capecitabine, gemcitabine, eribulin and vinorelbine).
The effect of severe renal impairment (CLcr 15 to 29 mL/min), end-stage renal disease with or without hemodialysis, and moderate (total bilirubin >1.5 to ≤3 ULN and any AST) or severe hepatic impairment (total bilirubin >3 ULN and any AST) on margetuximab-cmkb PK is unknown.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies have not been performed to evaluate carcinogenic or mutagenic potential of margetuximab-cmkb.
Animal fertility studies have not been conducted with margetuximab-cmkb. In repeat-dose toxicity studies of up to 13-week duration, margetuximab-cmkb had no effect on male and female reproductive organs in sexually mature cynomolgus monkeys.
14. Clinical Studies
14.1 Metastatic Breast Cancer
The efficacy of MARGENZA plus chemotherapy was evaluated in SOPHIA (NCT02492711), a randomized, multicenter, open-label trial of 536 patients with IHC 3+ or ISH-amplified HER2+ metastatic breast cancer who had received prior treatment with other anti-HER2 therapies. Patients were randomized (1:1) to MARGENZA plus chemotherapy or trastuzumab plus chemotherapy. Randomization was stratified by chemotherapy choice (capecitabine, eribulin, gemcitabine, or vinorelbine), number of lines of therapy in the metastatic setting (≤2, >2), and number of metastatic sites (≤2, >2). Patients were required to have progressed on or after the most recent line of therapy. Prior radiotherapy and hormonal therapy were allowed. Patients received MARGENZA intravenously at a dose of 15 mg/kg every 3 weeks administered over 120 minutes for the initial administration and then over 30 to 120 minutes thereafter. Trastuzumab was given intravenously at an initial dose of 8 mg/kg over 90 minutes, followed by 6 mg/kg over 30 minutes every 3 weeks thereafter. Patients were treated with MARGENZA or trastuzumab in combination with chemotherapy until disease progression or unacceptable toxicity.
Major efficacy outcome measures were progression-free survival (PFS) by blinded independent central (BICR) review and overall survival (OS) of MARGENZA plus chemotherapy, compared with trastuzumab plus chemotherapy. Additional efficacy outcome measures were objective response rate (ORR) and duration of response (DOR) assessed by BICR.
The median age was 56 years (range: 27-86); 78% of patients were <65 years. The majority of patients were female (99.4%), and the majority were White (80%). Patients had an ECOG performance status of 0 (58%) or 1 (42%) at baseline. Forty seven percent had visceral disease, 57% had bone metastases, and 13% had brain metastases. Sixty percent were hormone receptor positive. The median number of prior lines of therapy in the locally advanced/metastatic setting was 2 (range: 1-4). All study patients had previously received trastuzumab, all but 1 patient had previously received pertuzumab, and 91% had previously received ado-trastuzumab emtansine.
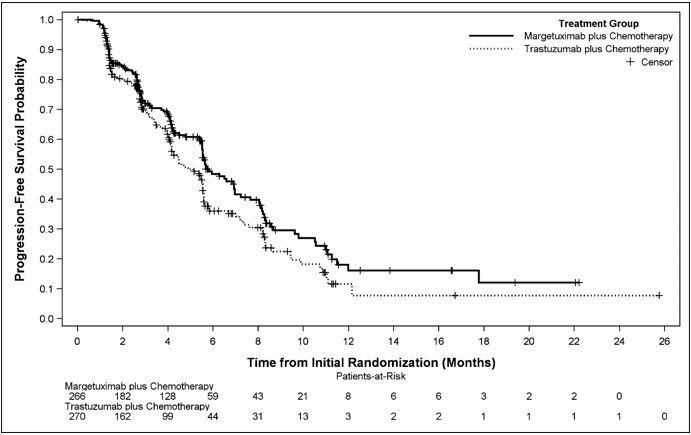
Efficacy results are summarized in Table 3 and Figure 1.
Table 3. Efficacy Results in SOPHIA:
| MARGENZA + Chemotherapy (n=266) | Trastuzumab + Chemotherapy (n=270) | |
|---|---|---|
| Progression-free Survival* | ||
| Number of events (%) | 130 (48.9) | 135 (50.0) |
| Disease progression | 118 (44.4) | 125 (46.3) |
| Death | 12 (4.5) | 10 (3.7) |
| Median, months (95% CI)† | 5.8 (5.5, 7.0) | 4.9 (4.2, 5.6) |
| Hazard Ratio (HR) (95% CI)‡ | 0.76 (0.59, 0.98) | |
| p-value§ | 0.033 | |
| Overall Survival | ||
| Number of events (%) | 194 (72.9) | 191 (70.7) |
| Median, months (95% CI)† | 21.6 (18.9, 25.1) | 21.9 (18.7, 24.2) |
| Hazard Ratio (HR) (95% CI)‡ | 0.95 (0.77, 1.17) | |
| p-value§ | 0.620¶ | |
| Objective Response for Patients with Measurable Disease* | (n=262) | (n=262) |
| Confirmed Objective Response Rate (95% CI) | 22 (17, 27) | 16 (12, 20) |
| Duration of Objective Response | (n=58) | (n=42) |
| Median (months) (95% CI)† | 6.1 (4.1, 9.1) | 6.0 (4.0, 6.9) |
CI: confidence interval; n: number of patients.
* Assessed per BICR.
† Based on Kaplan-Meier estimates.
‡ Based on stratified Cox Model.
§ p-value based on 2-sided stratified log rank test.
¶ Not statistically significant
Figure 1. Kaplan-Meier Curve for Progression-Free Survival in SOPHIA:
Results for investigator-assessed PFS were similar to the independent blinded PFS results.
Consistent PFS results were observed across patient subgroups defined by study stratification factors (chemotherapy choice, number of lines of therapy in the metastatic setting, and number of metastatic sites).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.