Trandolapril and Verapamil Hydrochloride Ref.[110106] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
Trandolapril and verapamil hydrochloride extended-release tablets combine a slow release formulation of a calcium channel blocker, verapamil hydrochloride, USP, and an immediate release formulation of an angiotensin converting enzyme inhibitor, trandolapril, USP.
Verapamil Component
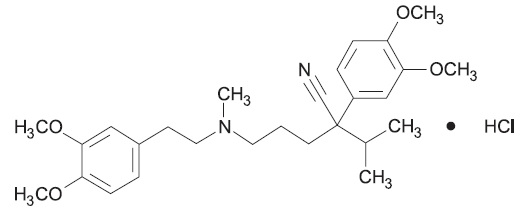
Verapamil hydrochloride, USP is chemically described as benzeneacetonitrile, α [3-[[2-(3,4-dimethoxyphenyl) -ethyl] methylamino] propyl] -3,4-dimethoxy-α -(1-methylethyl)-, monohydrochloride, (±). Its molecular formula is C27H38N2O4 • HCl and its structural formula is:
Verapamil hydrochloride, USP is a white or practically white crystalline powder, with a molecular weight of 491.06 g/mol. It is soluble in water, freely soluble in chloroform, sparingly soluble in alcohol and practically insoluble in ether. It is practically odorless and has a bitter taste.
Trandolapril Component
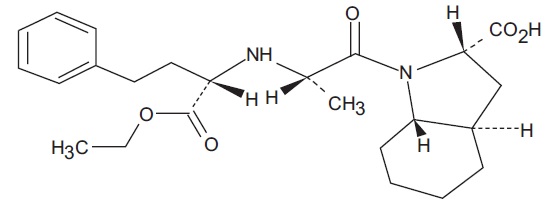
Trandolapril, USP is the ethyl ester prodrug of a nonsulfhydryl angiotensin converting enzyme (ACE) inhibitor, trandolaprilat. It is chemically described as (2S,3aR,7aS)1[(S)2[[(S)1(EthoxyCarbonyl)-3-phenylpropyl] amino]Propanoyl]octahydro-1H-indole-2-carboxylic acid. Its molecular formula is C24H34N2O5 and its structural formula is:
Trandolapril, USP is a white or almost white powder with a molecular weight of 430.54 g/mol. It is practically insoluble in water; freely soluble in methylene chloride; sparingly soluble in absolute alcohol.
Trandolapril and verapamil hydrochloride extended-release tablets are formulated for oral administration, containing verapamil hydrochloride, USP as a controlled release formulation and trandolapril, USP as an immediate release formulation. The tablet strengths are trandolapril and verapamil hydrochloride extended-release tablets 1 mg/240 mg, trandolapril and verapamil hydrochloride extended-release tablets 2 mg/180 mg, trandolapril and verapamil hydrochloride extended-release tablets 2 mg/240 mg, and trandolapril and verapamil hydrochloride extended-release tablets 4 mg/240 mg. The tablets also contain the following ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, ferric oxide red, hypromellose, lactose monohydrate, povidone, sodium alginate, sodium stearyl fumarate, magnesium stearate, and microcrystalline cellulose. The film coating contains: 1 mg/240 mg – hypromellose, titanium dioxide, and polyethylene glycol; 2 mg/180 mg – hypromellose, titanium dioxide, polyethylene glycol, iron oxide red, and FD&C blue #2; 2 mg/240 mg – hypromellose, titanium dioxide, polyethylene glycol, iron oxide yellow, iron oxide black, and iron oxide red; 4 mg/240 mg – hypromellose, titanium dioxide, polyethylene glycol, iron oxide yellow, iron oxide red, and iron oxide black.
| How Supplied |
|---|
|
Trandolapril and Verapamil Hydrochloride Extended-Release Tablets, 1 mg/240 mg are supplied as white to pinkish white colored, oval, biconvex, film-coated tablets with ‘294’ debossed on one side and plain on the other side containing 1 mg trandolapril, USP in an immediate-release form and 240 mg verapamil hydrochloride, USP in an extended-release form. NDC 68462-294-90 — bottles of 90 NDC 68462-294-01 — bottles of 100 NDC 68462-294-10 — bottles of 1000 Trandolapril and Verapamil Hydrochloride Extended-Release Tablets, 2 mg/180 mg are supplied as pink colored, oval, biconvex, film-coated tablets with ‘295’ debossed on one side and plain on the other side containing 2 mg trandolapril, USP in an immediate-release form and 180 mg verapamil hydrochloride, USP in an extended-release form. NDC 68462-295-90 — bottles of 90 NDC 68462-295-01 — bottles of 100 NDC 68462-295-10 — bottles of 1000 Trandolapril and Verapamil Hydrochloride Extended-Release Tablets, 2 mg/240 mg are supplied as cream colored, oval, biconvex, film-coated tablets with ‘296’ debossed on one side and plain on the other side containing 2 mg trandolapril, USP in an immediate-release form and 240 mg verapamil hydrochloride, USP in an extended-release form. NDC 68462-296-90 — bottles of 90 NDC 68462-296-01 — bottles of 100 NDC 68462-296-10 — bottles of 1000 Trandolapril and Verapamil Hydrochloride Extended-Release Tablets, 4 mg/240 mg are supplied as brown colored, oval, biconvex, film-coated tablets with ‘G38’ debossed on one side and plain on the other side containing 4 mg trandolapril, USP in an immediate-release form and 240 mg verapamil hydrochloride, USP in an extended-release form. NDC 68462-329-01 — bottles of 100 NDC 68462-329-10 — bottles of 1000 Dispense in well-closed container with safety closure. Manufactured by: Glenmark Pharmaceuticals Ltd., Colvale-Bardez, Goa 403513, India Manufactured for: Glenmark Pharmaceuticals Inc., USA, Mahwah, NJ 07430 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.