EYLEA 40 mg/mL Solution for injection in pre-filled syringe Ref.[110840] Active ingredients: Aflibercept
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Bayer AG, 51368 Leverkusen, Germany
4.1. Therapeutic indications
Eylea is indicated for adults for the treatment of
- neovascular (wet) age-related macular degeneration (AMD) (see section 5.1),
- visual impairment due to macular oedema secondary to retinal vein occlusion (branch RVO or central RVO) (see section 5.1),
- visual impairment due to diabetic macular oedema (DME) (see section 5.1),
- visual impairment due to myopic choroidal neovascularisation (myopic CNV) (see section 5.1).
EYLEA is indicated in preterm infants for the treatment of
- retinopathy of prematurity (ROP) with zone I (stage 1+, 2+, 3 or 3+), zone II (stage 2+ or 3+) or AP-ROP (aggressive posterior ROP) disease.
4.2. Posology and method of administration
Eylea is for intravitreal injection only.
Eylea must only be administered by a qualified physician experienced in administering intravitreal injections.
Posology
wet AMD
The recommended dose for Eylea is 2 mg aflibercept, equivalent to 0.05 mL.
Eylea treatment is initiated with one injection per month for three consecutive doses. The treatment interval is then extended to two months.
Based on the physician’s judgement of visual and/or anatomic outcomes, the treatment interval may be maintained at two months or further extended using a treat-and-extend dosing regimen, where injection intervals are increased in 2- or 4-weekly increments to maintain stable visual and/or anatomic outcomes.
If visual and/or anatomic outcomes deteriorate, the treatment interval should be shortened accordingly.
There is no requirement for monitoring between injections. Based on the physician’s judgement the schedule of monitoring visits may be more frequent than the injection visits.
Treatment intervals greater than four months or shorter than 4 weeks between injections have not been studied (see section 5.1).
Macular oedema secondary to RVO (branch RVO or central RVO)
The recommended dose for Eylea is 2 mg aflibercept equivalent to 0.05 mL. After the initial injection, treatment is given monthly. The interval between two doses should not be shorter than one month.
If visual and anatomic outcomes indicate that the patient is not benefiting from continued treatment, Eylea should be discontinued.
Monthly treatment continues until maximum visual acuity is achieved and/or there are no signs of disease activity. Three or more consecutive, monthly injections may be needed.
Treatment may then be continued with a treat-and-extend regimen with gradually increased treatment intervals to maintain stable visual and/or anatomic outcomes, however there are insufficient data to conclude on the length of these intervals. If visual and/or anatomic outcomes deteriorate, the treatment interval should be shortened accordingly.
The monitoring and treatment schedule should be determined by the treating physician based on the individual patient’s response.
Monitoring for disease activity may include clinical examination, functional testing or imaging techniques (e.g. optical coherence tomography or fluorescein angiography).
Diabetic macular oedema
The recommended dose for Eylea is 2 mg aflibercept equivalent to 0.05 mL.
Eylea treatment is initiated with one injection per month for five consecutive doses, followed by one injection every two months.
Based on the physician’s judgement of visual and/or anatomic outcomes, the treatment interval may be maintained at 2 months or individualized, such as with a treat-and-extend dosing regimen, where the treatment intervals are usually increased by 2-week increments to maintain stable visual and/or anatomic outcomes. There are limited data for treatment intervals longer than 4 months. If visual and/or anatomic outcomes deteriorate, the treatment interval should be shortened accordingly. Treatment intervals shorter than 4 weeks have not been studied (see section 5.1).
The schedule for monitoring should be determined by the treating physician.
If visual and anatomic outcomes indicate that the patient is not benefiting from continued treatment, Eylea should be discontinued.
Myopic choroidal neovascularisation
The recommended dose for Eylea is a single intravitreal injection of 2 mg aflibercept equivalent to 0.05 mL.
Additional doses may be administered if visual and/or anatomic outcomes indicate that the disease persists. Recurrences should be treated as a new manifestation of the disease.
The schedule for monitoring should be determined by the treating physician.
The interval between two doses should not be shorter than one month.
Retinopathy of prematurity (ROP)
The recommended dose for Eylea is a single intravitreal injection of 0.4 mg aflibercept equivalent to 0.01 mL.
Treatment of ROP is initiated with a single injection per eye and may be given bilaterally on the same day. In total, up to 2 injections per eye may be administered within 6 months of treatment initiation if there are signs of disease activity. The treatment interval between the 2 doses injected into the same eye should be at least 4 weeks.
Special populations
Hepatic and/or renal impairment
No specific studies in patients with hepatic and/or renal impairment have been conducted with Eylea.
Available data do not suggest a need for a dose adjustment with Eylea in these patients (see section 5.2).
Elderly population
No special considerations are needed. There is limited experience in patients older than 75 years with DME.
Paediatric population
The safety and efficacy of Eylea in children and adolescents below 18 years of age for indications other than ROP have not been established (see section 4.4). There is no relevant use of Eylea in the paediatric population for the indications of wet AMD, CRVO, BRVO, DME and myopic CNV.
Method of administration
Intravitreal injections must be carried out according to medical standards and applicable guidelines by a qualified physician experienced in administering intravitreal injections. In general, adequate anaesthesia and asepsis, including topical broad spectrum microbicide (e.g. povidone iodine applied to the periocular skin, eyelid and ocular surface), have to be ensured. Surgical hand disinfection, sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent) are recommended.
Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure. Appropriate monitoring may consist of a check for perfusion of the optic nerve head or tonometry. If required, sterile equipment for paracentesis should be available.
Following intravitreal injection, adult patients should be instructed to report any symptoms suggestive of endophthalmitis (e.g. eye pain, redness of the eye, photophobia, blurring of vision) without delay. Patients with ROP should be observed by healthcare professionals for any signs suggestive of endophthalmitis (e.g. redness/irritation of the eye, ocular discharge, lid swelling, photophobia). Parents and caregivers should also be instructed to observe and report any signs suggestive of endophthalmitis without delay.
Each pre-filled syringe should only be used for the treatment of a single eye. Extraction of multiple doses from a pre-filled syringe may increase the risk of contamination and subsequent infection.
Adults
The pre-filled syringe contains more than the recommended dose of 2 mg aflibercept (equivalent to 0.05 mL solution for injection). The extractable volume of the syringe is the amount that can be expelled from the syringe and is not to be used in total. For the Eylea pre-filled syringe, the extractable volume is at least 0.09 mL. The excess volume must be expelled before injecting the recommended dose (see section 6.6).
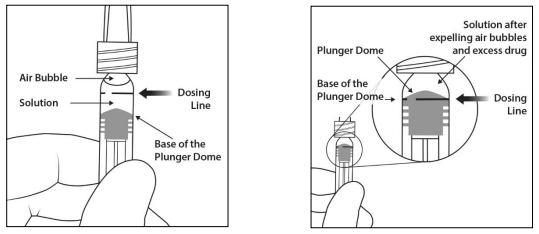
Injecting the entire volume of the pre-filled syringe could result in overdose. To expel the air bubbles along with excess medicinal product, slowly depress the plunger to align the base of the plunger dome (not the tip of the dome) with the dosing line on the syringe (equivalent to 0.05 mL i.e. 2 mg aflibercept) (see sections 4.9 and 6.6).
The injection needle should be inserted 3.5-4.0 mm posterior to the limbus into the vitreous cavity, avoiding the horizontal meridian and aiming towards the centre of the globe. The injection volume of 0.05 mL is then delivered; a different scleral site should be used for subsequent injections.
After injection any unused product must be discarded.
Paediatric population
For treatment of preterm infants, the PICLEO paediatric dosing device in combination with the prefilled syringe must be used for administration of a single dose of 0.4 mg aflibercept (equivalent to 0.01 mL solution for injection) (see section 6.6).
The injection needle should be inserted into the eye 1.0 to 2.0 mm from the limbus with the needle pointing towards the optic nerve.
After injection any unused product must be discarded.
For handling of the medicinal product before administration, see section 6.6.
4.9. Overdose
In clinical trials, doses of up to 4 mg in monthly intervals have been used and isolated cases of overdoses with 8 mg occurred.
Overdosing with increased injection volume may increase intraocular pressure. Therefore, in case of overdose, intraocular pressure should be monitored and if deemed necessary by the treating physician, adequate treatment should be initiated (see section 6.6).
6.3. Shelf life
2 years.
6.4. Special precautions for storage
Store in a refrigerator (2°C to 8°C).
Do not freeze.
Store in the original package in order to protect from light.
The unopened blister may be stored outside the refrigerator below 25°C for up to 24 hours. After opening the blister, proceed under aseptic conditions.
6.5. Nature and contents of container
Solution in pre-filled syringe (type I glass) marked with a dosing line, with a plunger stopper (elastomeric rubber) and a Luer lock adaptor with a tip cap (elastomeric rubber). Each pre-filled syringe contains an extractable volume of at least 0.09 mL. Pack size of 1 pre-filled syringe.
6.6. Special precautions for disposal and other handling
The pre-filled syringe is for single use in one eye only. Extraction of multiple doses from a pre-filled syringe may increase the risk of contamination and subsequent infection. Do not open the sterile pre-filled syringe blister outside the clean administration room. Any unused medicinal product or waste material should be disposed of in accordance with local requirements
The pre-filled syringe contains more than the recommended dose of 2 mg aflibercept (equivalent to 0.05 mL) for adult patients and 0.4 mg aflibercept (equivalent to 0.01 mL) for preterm infants. See following sections “Use in the adult population” and “Use in the paediatric population”.
The solution should be inspected visually for any foreign particulate matter and/or discolouration or any variation in physical appearance prior to administration. In the event of either being observed, discard the medicinal product.
For the intravitreal injection, a 30 G x ½ inch injection needle should be used.
Instructions for use of pre-filled syringe
Use in the paediatric population
To prepare the pre-filled syringe for administration to preterm infants, follow the steps 1 and 2 below and then adhere to the instructions for use included in the package of the PICLEO paediatric dosing device.
Use in the adult population
To prepare the pre-filled syringe for administration to adults, follow all steps below.
1. When ready to administer Eylea, open the carton and remove the sterilised blister. Carefully peel open the blister ensuring the sterility of its contents. Keep the syringe in the sterile tray until you are ready for assembly.
2. Using aseptic technique, remove the syringe from the sterilised blister.
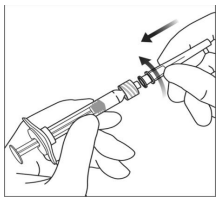
3. To remove the syringe cap, hold the syringe in one hand while using the other hand to grasp the syringe cap with the thumb and fore finger. Please note: You should twist off (do not snap off) the syringe cap.
4. To avoid compromising the sterility of the product, do not pull back on the plunger.
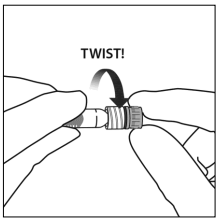
5. Using aseptic technique, firmly twist the injection needle onto the Luer-lock syringe tip.
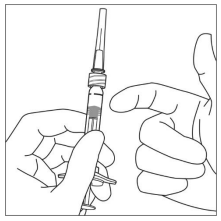
6. Holding the syringe with the needle pointing up, check the syringe for bubbles. If there are bubbles, gently tap the syringe with your finger until the bubbles rise to the top.
7. The excess volume must be discarded prior to administration. Eliminate all bubbles and expel excess medicinal product by slowly depressing the plunger to align the base of the plunger dome (not the tip of the dome) with the dosing line on the syringe (equivalent to 0.05 mL i.e. 2 mg aflibercept).
Note: This accurate positioning of the plunger is very important, because incorrect plunger positioning can lead to delivering more or less than the labelled dose.
8. Inject while pressing the plunger carefully and with constant pressure. Do not apply additional pressure once the plunger has reached the bottom of the syringe. Do not administer any residual solution observed in the syringe.
9. The pre-filled syringe is for single use only. Extraction of multiple doses from a pre-filled syringe may increase the risk of contamination and subsequent infection. Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.