PALYNZIQ Solution for injection Ref.[113789] Active ingredients: Pegvaliase
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: BioMarin International Limited, Shanbally, Ringaskiddy, County Cork, Ireland, P43 R298
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other alimentary tract and metabolism products, Enzymes
ATC code: A16AB19
Pegvaliase is rAvPAL conjugated with linear 20 kDa NHS-PEG at a degree of substitution of 28 to 44 moles of polymer/mole of protein. The average molecular mass is approximately 1,000 kDa of which the protein moiety constitutes approximately 248 kDa.
Mechanism of action
Pegvaliase is a PEGylated recombinant phenylalanine ammonia lyase enzyme that converts phenylalanine to ammonia and trans-cinnamic acid that are primarily eliminated by liver metabolism.
Clinical efficacy and safety
The effects of Palynziq in the treatment of PKU have been demonstrated in patients with phenylketonuria in Study 301, an open-label study to initiate Palynziq treatment, and Study 302, a follow-on study for efficacy assessment.
Study 301: Treatment initiation (Induction and Titration)
Study 301 an open-label randomised (1:1), multi-centre study of patients with PKU to assess the safety and tolerability of self-administered Palynziq in an induction/titration/maintenance dose regimen. The 261 enrolled patients were aged 16 to 55 years (mean: 29 years) and had a baseline mean blood phenylalanine of 1233 micromol/l. At treatment initiation, 253 (97%) patients had inadequate blood phenylalanine control (blood phenylalanine levels above 600 micromol/l) and 8 patients had blood phenylalanine levels less than or equal to 600 micromol/l. Patients previously treated with sapropterin were required to discontinue treatment at least 14 days prior to first dose of Palynziq. At baseline, 149 (57%) patients were receiving part of their total protein intake from medical food and 41 out of 261 (16%) patients were on a phenylalanine-restricted diet (defined as receiving greater than 75% of total protein intake from medical food). Patients initiated Palynziq treatment with an induction regimen (2.5 mg once week for 4 weeks) and were titrated in a stepwise manner (increased dose and frequency) to reach their randomised target dose of 20 mg once daily or 40 mg once daily. The duration of titration varied among patients and was based on patient tolerability (up to 30 weeks). For this study, the maintenance period was defined as at least 3 weeks dosing at randomised 20 mg or 40 mg once daily.
Of the 261 enrolled patients, 195 (75%) patients reached their randomised maintenance dose (103 patients in the 20 mg once daily arm, 92 patients in the 40 mg once daily arm). Patients in the 20 mg once daily randomised arm reached their maintenance dose at a median time of 10 weeks (range: 9 to 29 weeks) and patients in the 40 mg once daily arm reached their maintenance dose at a median time of 11 weeks (range: 10 to 33 weeks). Of the 261 patients who enrolled in Study 301, 152 patients continued to the eligibility period of Study 302, and 51 patients continued directly from Study 301 into the long-term extension period of Study 302.
Study 302: Efficacy assessment
Study 302 was a follow-on study (from Study 301) and included: an open label eligibility period; a double-blind, placebo-controlled randomised discontinuation trial period (RDT), and a long-term open-label extension period.
Eligibility period
A total of 164 previously-treated Palynziq patients (152 patients from Study 301, and 12 patients from other Palynziq trials) continued treatment for up to 13 weeks.
Of the 164 patients that entered the eligibility period of Study 302, 86 patients met the eligibility criterion (achieved at least 20% mean blood phenylalanine reduction from pre-treatment baseline at their randomised dose within 13 weeks) and continued to the RDT, 12 patients discontinued treatment, and 57 patients did not enter the RDT and continued Palynziq treatment in the long-term extension period of Study 302, where they were allowed to increase dose.
Randomised discontinuation trial (RDT) period
In the double-blind, placebo-controlled RDT, patients were randomised in a 2:1 ratio to either continue their randomised dosing (20 mg/day or 40 mg/day) or receive matching placebo for 8 weeks.
The primary endpoint was change from RDT baseline to RDT Week 8 in blood phenylalanine levels. Palynziq-treated patients were able to maintain their blood phenylalanine reductions compared to the placebo patients whose blood phenylalanine levels returned to their pre-treatment baseline levels after 8 weeks (p<0.0001, see Table 3).
Table 3. LS Mean change from RDT baseline in blood phenylalanine concentration (micromol/l) at RDT Week 8 in patients with PKU (Study 302):
| Randomised study arm | Blood phenylalanine concentration (micromol/l) Mean (SD) | LS mean change from Study 302 RDT baseline to Week 8 (95% CI) | Treatment difference in LS mean change (95% CI) P-value2 | ||
|---|---|---|---|---|---|
| Pre- treatment baseline1 | Study 302 RDT baseline | Study 302 RDT Week 8 | |||
| Palynziq 20 mg once daily3 | 1450.2 (310.5) n=29 | 596.8 (582.8) n=29 | 553.0 (582.4) n=26 | -23.3 (-156.2, 109.7) | -973.0 (-1204.2, -741.9) p<0.0001 |
| Placebo 20 mg once daily4 | 1459.1 (354.7) n=14 | 563.9 (504.6) n=14 | 1509.0 (372.6) n=13 | 949.8 (760.4, 1139.1) | |
| Palynziq 40 mg once daily3 | 1185.8 (344.0) n=29 | 410.9 (440.0) n=29 | 566.3 (567.5) n=23 | 76.3 (-60.2, 212.8) | -588.5 (-830.1, -346.9) p<0.0001 |
| Placebo 40 mg once daily4 | 1108.9 (266.8) n=14 | 508.2 (363.7) n=14 | 1164.4 (343.3) n=10 | 664.8 (465.5, 864.1) | |
1 Blood phenylalanine level prior to initiating treatment with Palynziq.
2 Based on the mixed model repeated measures (MMRM) method, with treatment arm, visit, and treatment arm-by-visit interaction (the time profile of blood phenylalanine changes is assessed separately for each treatment arm) as factors adjusting for baseline blood phenylalanine concentration.
3 Nine patients were excluded from the Week 8 analysis from the Palynziq treatment arms (20 mg/day or 40 mg/day): 4 patients did not complete the RDT due to adverse events (1 patient discontinued treatment and 3 patients transitioned to the long-term extension period) and the remaining 5 patients who did not complete phenylalanine assessment within the window for Week 8 (day 43 to 56).
4 Five patients were excluded from the Week 8 analysis from the placebo arms (20 mg/day or 40 mg/day):
1 patient did not complete the RDT due to adverse event transitioned to the long-term extension period and the remaining 4 patients who did not complete phenylalanine assessment within the window for Week 8 (day 43 to 56).
Symptoms of inattention and mood were also evaluated during this period. No differences were observed in inattention and mood between patients randomised to placebo versus those randomised to Palynziq during this 8-week duration.
Long-term extension period
Patients continued Palynziq treatment in the long-term open-label extension period and dose was adjusted (5, 10, 20, 40 and 60 mg/day) by the physician to achieve further blood phenylalanine reductions and maintain previously achieved phenylalanine levels.
Overall treatment experience from Study 301 and Study 302
At the time of completion of the studies, 188 out of the 261 patients received treatment for at least 1 year, 4 patients completed treatment, and 69 discontinued treatment in the first year. Of these 188 patients, 165 patients received treatment for at least 2 years, 22 patients discontinued in the second year, and 9 patients discontinued after 2 years of treatment. Of the 100 patients who discontinued treatment, 40 patients discontinued due to an adverse event, 29 patients discontinued due to patient decision, 10 patients discontinued due to physician decision, and 21 patients discontinued to other reasons (e.g. lost to follow-up, pregnancy, or protocol deviation).
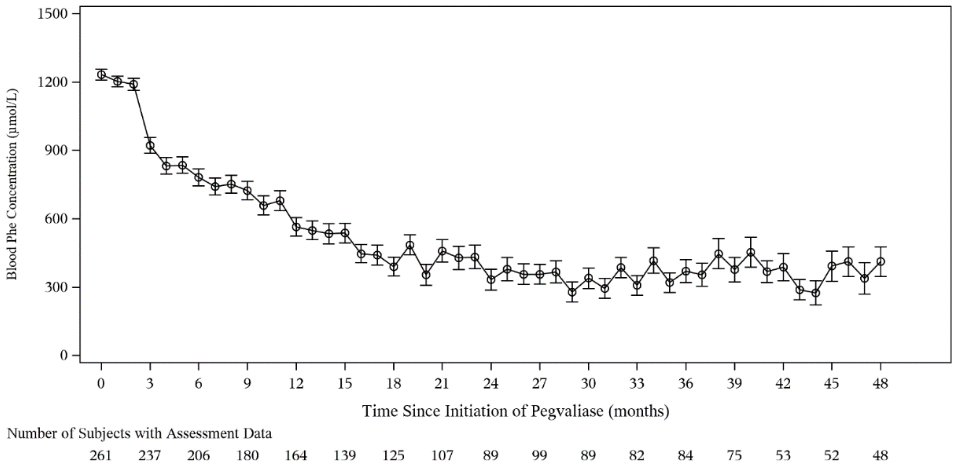
Efficacy results over time are presented in Table 4 and Figure 1.
Phenylalanine levels over time
Mean blood phenylalanine levels reduced from 1 233 micromol/l at baseline to 565 micromol/l at Month 12 (n=164) and 333 micromol/l at Month 24 (n=89), and these reductions in mean blood phenylalanine levels were maintained through Month 36 (371 micromol/l; n=84) (see Table 4 and Figure 1). Median change from baseline was -634 micromol/l at Month 12, -968 micromol/l at Month 24, and -895 micromol/l at Month 36.
ADHD inattention and PKU-POMS confusion over time
Symptoms of inattention were assessed using the inattention subscale of the investigator-rated Attention Deficient Hyperactivity Disorder Rating Scale (ADHD-RS IV). The ADHD-RS IV inattention subscale ranges from 0 to 27, higher scores indicate a greater degree of impairment, and a score below 9 indicates that the patient is asymptomatic (i.e. has a score that is in the normative range). Results for ADHD inattention subscale over time are shown in Table 4. Mean reduction (suggesting improvement) from baseline ADHD-RS inattention was above the minimally clinically important difference (MCID) for adults with ADHD (defined as a reduction of at least 5.2) at Month 18 (n=168; a reduction of 5.3), Month 24 (n=159; a reduction of 5.9) and Month 36 (n=142; a reduction of 6.6). In patients with baseline ADHD inattention scores > 9 (suggesting symptoms of inattention at baseline), mean reduction in ADHD inattention score from baseline (suggesting improvement) was above the MCID estimated for adults with ADHD at Month 12 (n=80; a reduction of 7.8), Month 18 (n=78; a reduction of 8.9), Month 24 (n=76; a reduction of 9.6) and Month 36 (n=66; a reduction of 10.7).
Symptoms of mood (confusion, fatigue, depression, tension-anxiety, vigour, and anger domains) were evaluated using the Profile of Mood States (POMS) tool that has been modified to be specific to PKU (PKU-POMS). The PKU-POMS confusion subscale (ranging from 0 to 12 points with higher scores indicating greater degree of impairment) was considered most sensitive to changes in blood phenylalanine levels. Results for PKU-POMS confusion subscale over time are shown in Table 4. Mean change from baseline PKU-POMS confusion subscale (suggesting improvement) was above MCID (defined as a reduction of at least 1) at Month 12 (n=130; a reduction of 1.6), Month 18 (n=123; a reduction of 2), Month 24 (n=116; a reduction of 2.2) and Month 36 (n=103; a reduction of 2.2).
Changes in protein intake from intact food over time
Median protein intake from intact food increased at Month 12 (4 g increase from baseline), Month 24 (14 g increase from baseline) and Month 36 (20 g increase from baseline).
Figure 1. Mean (SE) phenylalanine levels over time:
Table 4. Efficacy results at Month 12, Month 18, Month 24 and Month 36 in Palynziq-treated patients:
| Baseline | Month 12 | Month 18 | Month 24 | Month 36 | |
|---|---|---|---|---|---|
| Blood phenylalanine1 | |||||
| N | 261 | 1642 | 1252 | 892 | 842 |
| Mean (SD) blood phenylalanine (micromol/l) | 1 233 (386) | 565 (531) | 390 (469) | 333 (441) | 371 (459) |
| Change from baseline (micromol/l) Mean (SD) Median | - | -662 (588) -634 | -883 (565) -920 | -882 (563) -968 | -911 (563) -895 |
| ADHD inattention3 subscale (investigator-rated) | |||||
| N | 253 | 178 | 175 | 166 | 147 |
| Mean (SD) inattention score | 9.8 (6.1) | 5 (4.9) | 4.6 (4.7) | 4.3 (4.6) | 3.4 (4.5) |
| Change from baseline inattention score (n)4 Mean (SD) Median | - | n=172 -4.7 (5.6) -4 | n=168 -5.3 (5.9) -5 | n=159 -5.9 (6.1) -5 | n=142 -6.6 (6.1) -5 |
| ADHD inattention3 subscale (investigator-rated) with baseline score >9 | |||||
| N | 116 | 80 | 78 | 76 | 66 |
| Mean (SD) inattention score | 15.3 (4.1) | 7.6 (4.9) | 6.6 (5) | 5.9 (4.9) | 4.9 (5.3) |
| Change from baseline inattention score (n)4 Mean (SD) Median | - | n=80 -7.8 (5.5) -7 | n=78 -8.9 (5.8) -9 | n=76 -9.6 (5.9) -10 | n=66 -10.7 (6.0) -12 |
| PKU-POMS confusion3 subscale (self-rated) | |||||
| N | 170 | 181 | 178 | 168 | 152 |
| Mean (SD) confusion score | 4 (2.7) | 2.4 (2.1) | 2.1 (2.2) | 2 (2.1) | 1.9 (2.1) |
| Change from baseline confusion score (n)4 Mean (SD) Median | - | n=130 -1.6 (2.5) -1 | n=123 -2 (2.8) -2 | n=116 -2.2 (2.7) -2 | n=103 -2.2 (3.0) -2 |
| Protein intake from intact food (g) | |||||
| N | 250 | 160 | 111 | 83 | 80 |
| Mean (SD) | 39 (28) | 47 (29) | 50 (27) | 55 (27) | 66 (27) |
| Change from baseline protein intake (n)4 Mean (SD) Median | - | n=154 9 (25) 4 | n=106 12 (25) 9 | n=80 16 (27) 14 | n=78 24 (31) 20 |
1 Post-baseline phenylalanine values were mapped to the closest monthly visit (i.e. within a 1-month window).
2 Reflects number of patients who reached time point (Month 12/Month 18/Month 24/Month 36) of treatment at the time of the data cut-off and had a scheduled phenylalanine assessment for that time point.
3 Post-baseline ADHD-inattention/PKU-POMS confusion values were mapped to the closest 3-month visit (i.e. within a 3-month window).
4 Change from baseline was based on subjects with available measurements at both time points. Not all subjects had a baseline ADHD inattention score and POMS confusion score taken at the start of the study.
Impact blood phenylalanine reduction on ADHD inattention and PKU-POMS confusion
An analysis of ADHD inattention and PKU-POMS confusion subscales by change in blood phenylalanine from baseline quartiles showed that patients with the largest phenylalanine reductions experienced the greatest improvements in ADHD inattention and PKU-POMS confusion subscales.
Paediatric population
No data are available in paediatric patients less than 16 years of age.
Of the 261 patients in Study 301, 11 patients were aged between 16 and 18 years at enrolment. All 11 patients had inadequate blood phenylalanine control (blood phenylalanine levels above 600 micromol/l) at baseline. These patients received the same induction/titration/maintenance regimen as patients aged 18 years and older in this study. Mean (SD) change from baseline was 20 (323) micromol/l at Month 12 (n=9), -460 (685) micromol/l at Month 24 (n=5), and -783 (406) micromol/l at Month 36 (n=5). Of the 11 patients initially enrolled in Study 301, 3 patients reached blood phenylalanine levels ≤600 micromol/l by 12 months, 7 patients reached this threshold by 24 months, and 8 patients reached this threshold by 36 months.
The European Medicines Agency has deferred the obligation to submit the results of studies with Palynziq in one or more subsets of the paediatric population for the treatment of hyperphenylalaninaemia (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Pegvaliase is a PEGylated recombinant phenylalanine ammonia lyase (rAvPAL), derived from the cyanobacterium Anabaena variabilis expressed in Escherichia coli. The purpose of the PEGylation of rAvPAL is to reduce immune recognition of the rAvPAL bacterial protein and increase the half-life.
The pharmacokinetics of pegvaliase exhibit high inter-patient and intra-patient variability due to the heterogeneity of the immune response in adult patients with PKU. Immune response affects clearance and time to reach steady state. The immune response stabilises over 6 to 9 months of total treatment.
Absorption
Following a single subcutaneous dose (0.01, 0.03 or 0.1 mg/kg), pegvaliase is absorbed slowly with a median tmax of 3.5 to 4 days (individual range of 2.5 to 7 days). The bioavailability is not affected by the different injection sites on the body (see section 4.2). The absolute bioavailability in humans is unknown.
Distribution
Mean (SD) for apparent volume of distribution (Vz/F) at steady state following 20 mg and 40 mg doses was 26.4 L (64.8 L) and 22.2 L (19.7 L) respectively.
Biotransformation
Following cellular uptake, the metabolism of phenylalanine ammonia lyase (PAL) is expected to occur via catabolic pathways and be degraded into small peptides and amino acids; the PEG molecule is metabolically stable and expected to be separated from PAL protein and primarily eliminated by renal filtration.
Elimination
Pegvaliase is primarily cleared by immune-mediated mechanisms following repeat dosing. In clinical trials, anti-PAL, anti-PEG and anti-pegvaliase have been identified as IgG and IgM mainly. Relatively low titres of IgE has also been observed. In maintenance phase of the treatment, steady state is expected 4 to 24 weeks after maintenance dose started. A mean (SD) half-life at 20 mg and 40 mg were 47.3 hours (41.6 hours) and 60.2 hours (44.6 hours), respectively. Individual values for half-life range from 14 to 132 hours. The PEG molecule is expected to be primarily eliminated by renal filtration.
Linearity/nonlinearity
During dose escalation from 20 mg/day to 40 mg/day and 40 mg/day to 60 mg/day, a greater dose proportional increase in exposure was observed.
Specific populations
Analysis of pegvaliase concentration data from clinical trials indicated that body weight, gender and age did not have a notable effect on pegvaliase pharmacokinetics. No clinical trials have been conducted to evaluate the effect of renal or hepatic impairment on the pharmacokinetics of pegvaliase.
Exposure-effect
A PK/PD analysis using the Phase III data demonstrated an inverse pegvaliase exposure-phenylalanine response relationship, which could be influenced by dietary phenylalanine intake. At lower plasma pegvaliase Ctrough concentrations <10 000 ng/ml, patients with higher dietary phenylalanine intake tend to have higher blood phenylalanine levels compared to patients with the same Ctrough concentration and lower dietary phenylalanine intake, suggesting saturation of the enzyme (i.e. rAvPAL). At high pegvaliase Ctrough concentrations ≥10 000 ng/ml, the majority of the blood phenylalanine levels (97%) are ≤30 micromol/l, even when dietary phenylalanine intake is high. Therefore, a reduction in pegvaliase dose should be considered in patients experiencing hypophenylalaninaemia despite appropriate levels of protein intake (see section 4.2).
5.3. Preclinical safety data
Dose-dependent reductions in body weight gain attributed to decreased plasma phenylalanine levels to below normal levels in normal animals (monkeys, rats and rabbits) was observed in single and repeat dose toxicology studies as well as developmental and reproductive toxicity studies with pegvaliase. Decreased plasma phenylalanine and reduced body weight gain was reversible after cessation of treatment.
In cynomolgus monkeys, the incidence and severity of arterial inflammation was dose dependent and observed in a wide range of organs and tissues at clinically relevant exposures in the 4- and 39-week repeat-dose toxicology studies. The arterial inflammation observed in these studies involved small arteries and arterioles in a wide range of organs and tissues and in subcutaneous injection sites. Arteritis was attributed to the immune-mediated response associated with chronic administration of foreign protein to the animals. The vascular inflammation observed in these studies was reversible upon cessation of treatment.
In rats, dose dependent vacuolation attributed to pegvaliase treatment was observed at clinically relevant exposures in the 4- and 26-week repeat-dose toxicity studies in rats in multiple organs and tissues, but not in cynomolgus monkeys. No vacuolation was observed in the brain. Vacuoles in all tissues, with the exception of the kidney, resolved or were diminished by the end of the recovery period, suggesting partial reversibility. The vacuolation observed in these studies was not associated with any organ related toxicities as determined by clinical chemistry/urinalysis and histopathological analysis. The clinical significance of these findings and functional consequences are unknown.
Adverse reproductive and developmental effects of pegvaliase in rats and rabbits were dose dependent and included reduced implantation rate, smaller litter size, lower foetal weights, and increased foetal alterations. Additional findings in rabbits included increased abortions, foetal malformations and embryo/foetal lethality. These findings occurred in the presence of maternal toxicity (decreased body weights, decreased ovarian weights, and decreased food consumption) and were associated with markedly decreased maternal blood phenylalanine below normal levels in non-PKU animals. The contribution of maternal phenylalanine depletion to the incidence of embryo-foetal developmental effects was not evaluated.
In the peri/postnatal study, pegvaliase decreased pup weight, litter size, and survival of offspring during lactation, and delayed sexual maturation of offspring when administered daily in rats at 20 mg/kg subcutaneously. The effects in offspring were associated with maternal toxicity.
Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with pegvaliase. Based on its mechanism of action, pegvaliase is not expected to be tumorigenic.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.