BEOVU Solution for injection Ref.[9918] Active ingredients: Brolucizumab
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Brolucizumab is a human VEGF inhibitor. Brolucizumab binds to the three major isoforms of VEGF-A (e.g., VEGF110, VEGF121, and VEGF165), thereby preventing interaction with receptors VEGFR-1 and VEGFR-2. By inhibiting VEGF-A, brolucizumab suppresses endothelial cell proliferation, neovascularization, and vascular permeability.
12.2. Pharmacodynamics
Leakage of blood and fluid from choroidal neovascularization (CNV) may cause retinal thickening or edema. Reductions in central retinal subfield thickness (CST) were observed across all treatment arms.
12.3. Pharmacokinetics
Following a single intravitreal dose of 6 mg BEOVU to 25 AMD patients, the mean (range) serum Cmax of free brolucizumab (unbound to VEGF-A) was 49 ng/mL (9 to 548 ng/mL) and was attained at 24 hours post-dose. Brolucizumab concentrations were near or less than 0.5 ng/mL (lower limit of assay quantitation) at approximately 4 weeks after repeat dose administration and no accumulation in serum was observed in most patients.
Elimination
The estimated mean (± standard deviation) systemic half-life of brolucizumab is 4.4 days (± 2.0 days) after a single intravitreal dose.
Metabolism
Metabolism of brolucizumab has not been fully characterized. However, free brolucizumab is expected to undergo metabolism via proteolysis.
Excretion
The excretion of brolucizumab has not been fully characterized. However, free brolucizumab is expected to undergo target-mediated disposition and/or passive renal excretion.
Specific Populations
Following repeat intravitreal dose administration of 6 mg BEOVU, no differences in the systemic pharmacokinetics of brolucizumab were observed based on age (50 years and above), sex, or mild to moderate renal impairment (glomerular filtration rate (GFR) = 30 to 70 mL/min, estimated using the Modification of Diet in Renal Disease (MDRD) equation). The effect of severe renal impairment or any degree of hepatic impairment on the pharmacokinetics of BEOVU is unknown. As significant increases in serum brolucizumab exposures are not expected with intravitreal route of administration, no dosage adjustment is needed based on renal or hepatic impairment status.
Drug Interaction Studies
No studies evaluating the drug interaction potential of BEOVU have been conducted.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted on the carcinogenic or mutagenic potential of BEOVU. Based on the anti-VEGF mechanism of action, treatment with BEOVU may pose a risk to reproductive capacity [see Use in Specific Populations (8.3)].
14. Clinical Studies
14.1 Neovascular (Wet) Age-Related Macular Degeneration (AMD)
The safety and efficacy of BEOVU were assessed in two randomized, multi-center, double-masked, active-controlled studies (HAWK – NCT02307682 and HARRIER – NCT02434328) in patients with neovascular AMD. A total of 1817 patients were treated in these studies for two years (1088 on brolucizumab and 729 on control). Patient ages ranged from 50 to 97 years with a mean of 76 years.
In HAWK, patients were randomized in a 1:1:1 ratio to the following dosing regimens:
- brolucizumab 3 mg administered every 8 or 12 weeks after the first 3 monthly doses,
- brolucizumab 6 mg administered every 8 or 12 weeks after the first 3 monthly doses,
- aflibercept 2 mg administered every 8 weeks after the first 3 monthly doses.
In HARRIER, patients were randomized in a 1:1 ratio to the following dosing regimens:
- brolucizumab 6 mg administered every 8 or 12 weeks after the first 3 monthly doses,
- aflibercept 2 mg administered every 8 weeks after the first 3 monthly doses.
In both studies, after three initial monthly doses (Week 0, 4, and 8), treating physicians decided whether to treat each individual patient on an every 8 week or 12 week dosing interval guided by visual and anatomical measures of disease activity, although the utility of these measures has not been established. Patients on 12 week dosing intervals could be changed based on the same measures to an 8 week schedule after subsequent treatment visits. Any patient placed on an 8 week schedule, remained on the 8 week dosing interval until the end of the study. Protocol-specified visits in the initial three months occurred every 28 ± 3 days followed by every 28 ± 7 days for the remainder of the studies. Baseline anatomical measures may have contributed to the regimen selection because the majority of patients on the 12-week dosing schedule at the end of the trial had less baseline macular edema and/or smaller baseline lesions.
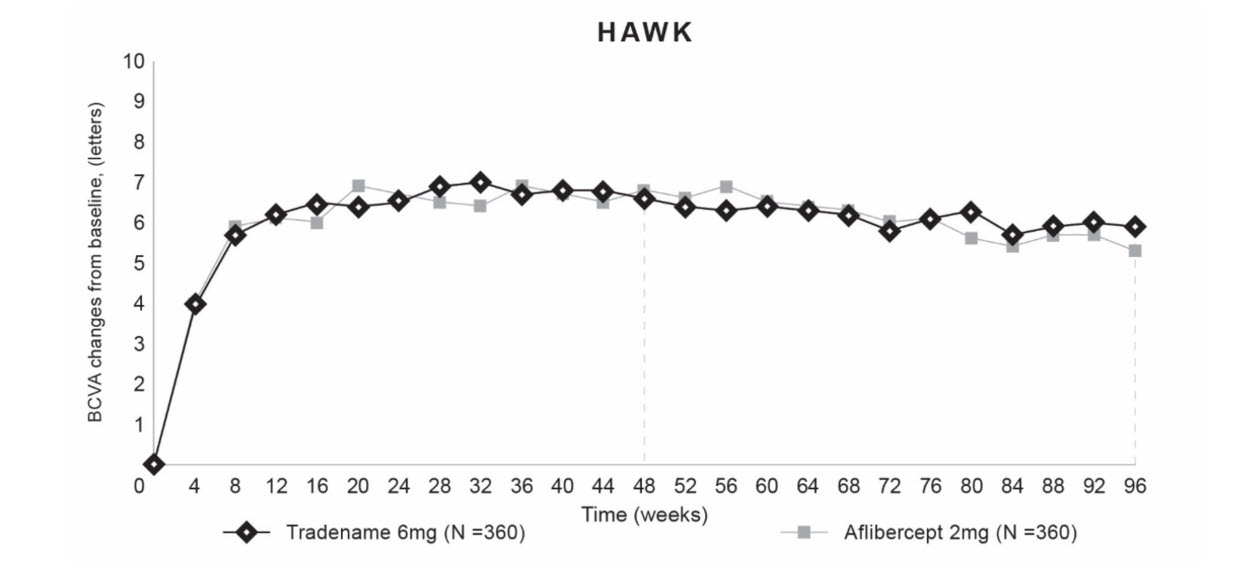
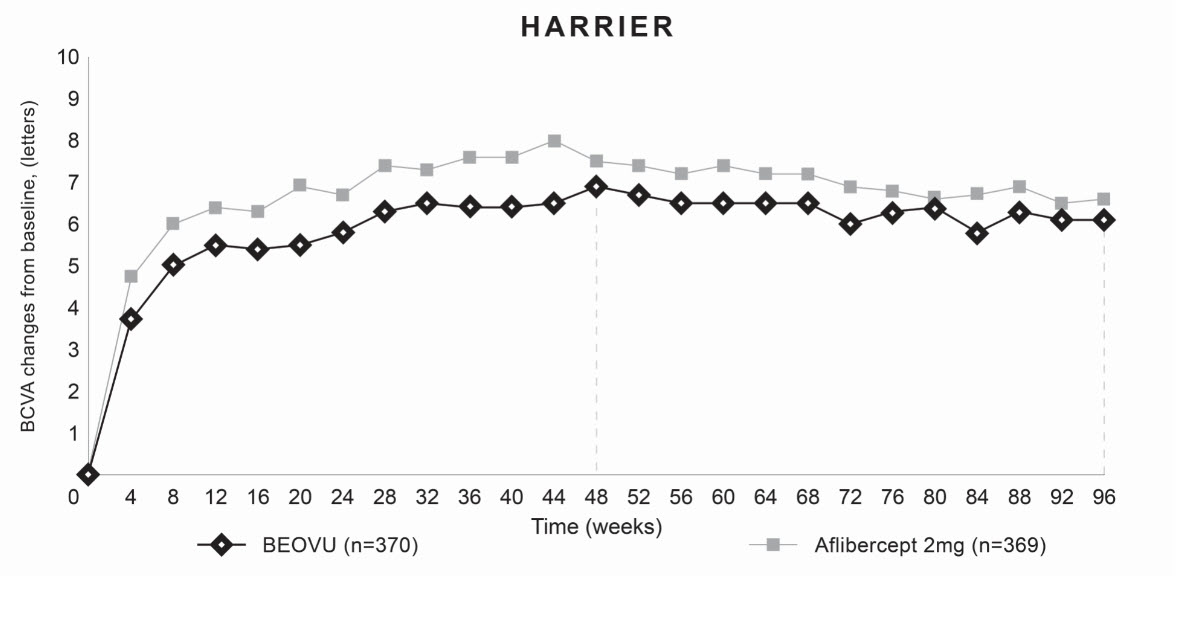
Both studies demonstrated efficacy in the primary endpoint defined as the change from baseline in Best Corrected Visual Acuity (BCVA) at Week 48, measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) Letter Score. In both studies, BEOVU treated patients had a similar mean change from baseline in BCVA as the patients treated with aflibercept 2 mg (fixed every 8 weeks). Detailed results of both studies are shown in Table 2 and Figures 5 and 6 below.
Table 2. Efficacy Outcomes at Week 48 and 96 in Phase 3 HAWK and HARRIER Studies:
| HAWK | HAWK | ||||||
|---|---|---|---|---|---|---|---|
| Efficacy outcome | At week | Beovu 6 mg (n=360) | Aflibercept 2 mg (n=360) | Difference (95% CI) brolucizumab-aflibercept | Beovu 6 mg (n=370) | Aflibercept 2 mg (n=369) | Difference (95% CI) brolucizumab-aflibercept |
| Mean change from baseline in BCVA (measured by ETDRS letters score) | 48 | 6,6 (SE=0,71) | 6,8 (SE=0,71) | -0,2 (-2,1, 1,8) P<0,0001 a) | 6,9 (SE=0,61) | 7,6 (SE=0,61) | -0,7 (-2,4, 1,0) P <0,0001a |
| 36-48b | 6,7 (SE=0,68) | 6,7 (SE=0,68) | 0,0 (-1,9, 1,9) P<0,0001a | 6,5 (SE=0,58) | 7,7 (SE=0,58) | -1,2 (-2,8, 0,4) P=0,0003a | |
| 96 | 5,9 (SE=0,78) | 5,3 (SE=0,78) | 0,5 (-1,6, 2,7) | 6,1 (SE=0,73) | 6,6 (SE=0,73) | -0,4 (-2,5,1,6) | |
| % of patients who gained at least 15 letters of vision | 48 | 33,6 | 25,4 | 8,2 (2,2, 15,0) | 29,3 | 29,9 | -0,6 (-7,1, 5,8) |
| 96 | 34,2 | 27,0 | 7,2 (1,4, 13,8) | 29,1 | 31,5 | -2,4 (-8,8, 4,1) | |
| % of patients who lost visual acuity (%) (≥15 letters of BCVA loss) | 48 | 6,4 | 5,5 | 0,9 (-2,7, 4,3) | 3,8 | 4,8 | -1,0 (-3,9, 2,2) |
| 96 | 8,1 | 7,4 | 0,7 (-3,6, 4,6) | 7,1 | 7,5 | -0,4 (-3,8, 3,3) | |
Abbreviations – BCVA: Best Corrected Visual Acuity; missing data are imputed using last observation carried forward (LOCF) method, ETDRS: Early Treatment Diabetic Retinopathy Study, SE: standard error.
Figure 5. Mean Change in Visual Acuity From Baseline to Week 96 in HAWK:
Figure 6. Mean Change in Visual Acuity From Baseline to Week 96 in HARRIER:
Through Week 48, 56% (HAWK) and 51% (HARRIER) of patients remained on BEOVU every 12 weeks. The proportion of patients who were maintained on every 12 week dosing through Week 96 was 45% and 39% in HAWK and HARRIER, respectively. The probability of remaining on every 12 week dosing from Week 20 to Week 48 was 85% and 82%, and from Week 48 to Week 96 was 82% and 75% in HAWK and HARRIER, respectively.
Treatment effects in evaluable subgroups (e.g., age, gender, race, baseline visual acuity) in each study were generally consistent with the results in the overall populations.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.