ABRYSVO Powder and solvent for solution for injection Ref.[51247] Active ingredients: RSV glycoprotein F antigen
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium
4.1. Therapeutic indications
Abrysvo is indicated for:
- Passive protection against lower respiratory tract disease caused by respiratory syncytial virus (RSV) in infants from birth through 6 months of age following maternal immunisation during pregnancy. See sections 4.2 and 5.1.
- Active immunisation of individuals 60 years of age and older for the prevention of lower respiratory tract disease caused by RSV.
The use of this vaccine should be in accordance with official recommendations.
4.2. Posology and method of administration
Posology
Pregnant individuals
A single dose of 0.5 mL should be administered between weeks 24 and 36 of gestation (see sections 4.4 and 5.1).
Individuals 60 years of age and older
A single dose of 0.5 mL should be administered.
Paediatric population
The safety and efficacy of Abrysvo in children (from birth to less than 18 years of age) have not yet been established. Limited data are available in pregnant adolescents and their infants (see section 5.1).
Method of administration
Abrysvo is for intramuscular injection into the deltoid region of the upper arm.
The vaccine should not be mixed with any other vaccines or medicinal products.
For instructions on reconstitution and handling of the medicinal product before administration, see section 6.6.
4.9. Overdose
Overdose with Abrysvo is unlikely due to its single dose presentation.
There is no specific treatment for an overdose with Abrysvo. In the event of an overdose, the individual should be monitored and provided with symptomatic treatment as appropriate.
6.3. Shelf life
2 years.
The unopened vial is stable for 5 days when stored at temperatures from 8°C to 30°C. At the end of this period Abrysvo should be used or discarded. This information is used to guide healthcare professionals in case of temporary temperature excursions only.
After reconstitution:
Abrysvo should be administered immediately after reconstitution or within 4 hours if stored between 15°C and 30°C. Do not freeze.
Chemical and physical in-use stability has been demonstrated for 4 hours between 15°C and 30°C. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
6.4. Special precautions for storage
Store in a refrigerator (2ºC-8ºC).
Do not freeze. Discard if the carton has been frozen.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Vial of antigens for Abrysvo (powder) and pre-filled syringe of solvent:
Powder for 1 dose in a vial (type 1 glass or equivalent) with a stopper (synthetic chlorobutyl rubber) and a flip off cap.
Solvent for 1 dose in a pre-filled syringe (type 1 glass) with a stopper (synthetic chlorobutyl rubber) and a tip cap (synthetic isoprene/bromobutyl blend rubber).
Vial adaptor.
Vial of antigens for Abrysvo (powder) and vial of solvent:
Powder for 1 dose in a vial (type 1 glass or equivalent) with a stopper (synthetic chlorobutyl rubber) and a flip off cap.
Solvent for 1 dose in a vial (type 1 glass or equivalent) with a stopper (bromobutyl rubber) and a flip off cap.
Pack sizes:
Pack containing 1 vial of powder (antigens), 1 pre-filled syringe of solvent, 1 vial adaptor with 1 needle or without needles (1 dose pack).
Pack containing 5 vials of powder (antigens), 5 pre-filled syringes of solvent, 5 vial adaptors with 5 needles or without needles (5 dose pack).
Pack containing 10 vials of powder (antigens), 10 pre-filled syringes of solvent, 10 vial adaptors with 10 needles or without needles (10 dose pack).
Pack containing 5 vials of powder (antigens) and 5 vials of solvent (5 dose pack).
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
For use of vial of antigens for Abrysvo (powder), pre-filled syringe of solvent and vial adaptor
Abrysvo must be reconstituted prior to administration by adding the entire contents of the pre-filled syringe of solvent to the vial containing the powder using the vial adaptor.
The vaccine must be reconstituted only with the solvent provided.
Preparation for administration
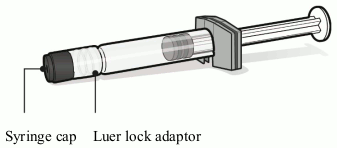
Pre-filled syringe containing solvent for Abrysvo:
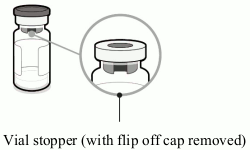
Vial containing antigens for Abrysvo (powder):
Vial adaptor:
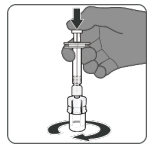
Step 1. Attach vial adaptor:
- Peel off the top cover from the vial adaptor packaging and remove the flip off cap from the vial.
- While keeping the vial adaptor in its packaging, centre over the vial’s stopper and connect with a straight downward push. Do not push the vial adaptor in at an angle as it may result in leaking. Remove the packaging.
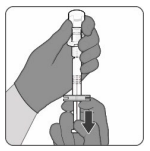
Step 2. Reconstitute the powder component (antigens) to form Abrysvo:
- For all syringe assembly steps, hold the syringe only by the Luer lock adaptor. This will prevent the Luer lock adaptor from detaching during use.
- Twist to remove the syringe cap, then twist to connect the syringe to the vial adaptor. Stop turning when you feel resistance.
- Inject the entire contents of the syringe into the vial. Hold the plunger rod down and gently swirl the vial until the powder is completely dissolved (approximately 1-2 minutes). Do not shake.
Step 3. Withdraw reconstituted vaccine:
- Invert the vial completely and slowly withdraw the entire contents into the syringe to ensure a 0.5 mL dose of Abrysvo.
- Twist to disconnect the syringe from the vial adaptor.
- Attach a sterile needle suitable for intramuscular injection.
The prepared vaccine is a clear and colourless solution. Visually inspect the vaccine for large particulate matter and discolouration prior to administration. Do not use if large particulate matter or discolouration is found.
For use of vial of antigens for Abrysvo (powder) and vial of solvent
The vial containing antigens for Abrysvo (powder) must be reconstituted only with the vial of solvent provided to form Abrysvo.
Preparation for administration
1. Using a sterile needle and sterile syringe, withdraw the entire contents of the vial containing the solvent and inject the entire contents of the syringe into the vial containing the powder.
2. Gently swirl the vial in a circular motion until the powder is completely dissolved. Do not shake.
3. Withdraw 0.5 mL from the vial containing the reconstituted vaccine.
The prepared vaccine is a clear and colourless solution. Visually inspect the vaccine for large particulate matter and discolouration prior to administration. Do not use if large particulate matter or discolouration is found.
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.