AFREZZA Inhalation Powder Ref.[49684] Active ingredients: Insulin (human)
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Insulin lowers blood glucose levels by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis in adipocytes, inhibits proteolysis, and enhances protein synthesis.
12.2. Pharmacodynamics
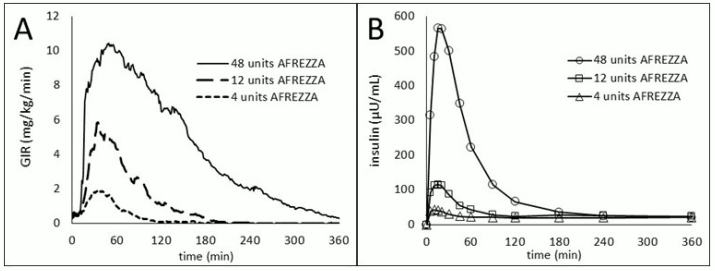
The time course of insulin action (i.e., glucose lowering) may vary considerably in different individuals or within the same individual. The average pharmacodynamic profile [i.e., glucose lowering effect measured by glucose infusion rate (GIR) over time in a euglycemic clamp study] for a single 4, 12, and 48 unit dose of AFREZZA in 30 patients with type 1 diabetes is shown in Figure 3(A), and key characteristics surrounding the timing of the effects are described in Table 4:
Table 4. Timing of insulin effect (i.e., mean pharmacodynamics effect) after administration for a single dose of 4, 12, and 48 units of AFREZZA in patients (N=30) with T1DM and corresponding to the data shown in Figure 3(A):
| Parameter for Insulin Effect | AFREZZA 4 units | AFREZZA 12 units | AFREZZA 48 units |
|---|---|---|---|
| Time to first measurable effect | ~12 minutes | ~12 minutes | ~12 minutes |
| Time to peak effect | ~35 minutes | ~45 minutes | ~55 minutes |
| Time for effect to return to baseline | ~90 minutes | ~180 minutes | ~270 minutes |
Figure 3. Mean Insulin Effect (Baseline-Corrected Glucose Infusion Rate; A) and Pharmacokinetic (Baseline-Corrected Serum Insulin Concentrations; B) profiles after Administration of AFREZZA 4, 12, and 48 units in Type 1 Diabetes Patients (N=30):
On average, the pharmacodynamics effect of AFREZZA, measured as area under the glucose infusion rate-time curve (AUC GIR) increased linearly with doses up to 48 units (106, 387, and 1581 mg/kg for 4, 12, and 48 units doses, respectively).
Intrapatient variability in AUC GIR and GIRmax is approximately 28% (95% CI 21-42%) and 27% (95% CI 20-40%), respectively.
12.3. Pharmacokinetics
Absorption
The pharmacokinetic profiles for orally inhaled AFREZZA 4, 12, and 48 units from a study in 30 patients with type 1 diabetes are shown in Figure 3(B). The time to maximum serum insulin concentration ranges from 10-20 minutes after oral inhalation of 4 to 48 units of AFREZZA. Serum insulin concentrations declined to baseline by approximately 60 to 240 minutes for these dose levels.
Disposition
Systemic insulin disposition (apparent terminal half-life) following oral inhalation of 4 to 48 units of AFREZZA was 120-206 minutes.
Variability
Intrapatient variability in insulin exposure measured by AUC and Cmax is approximately 16% (95% CI 12-23%) and 21% (95% CI 16-30%), respectively.
Metabolism and Elimination
The metabolism and elimination of AFREZZA are comparable to regular human insulin.
Carrier Particles
Clinical pharmacology studies showed that carrier particles [see Description (11.1)] are not metabolized and are eliminated unchanged in the urine following the lung absorption. Following oral inhalation of AFREZZA, a mean of 39% of the inhaled dose of carrier particles was distributed to the lungs and a mean of 7% of the dose was swallowed. The swallowed fraction was not absorbed from the GI tract and was eliminated unchanged in the feces.
Drug Interaction: Bronchodilators and Inhaled Steroids
Albuterol increased the AUC insulin administered by AFREZZA by 25% in patients with asthma. Effect of fluticasone on insulin exposures following AFREZZA administration has not been evaluated in patients with asthma; however, no significant change in insulin exposure was observed in a study in healthy volunteers. Frequent glucose monitoring and dose reduction may be necessary for AFREZZA if it is co-administered with albuterol.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104 week carcinogenicity study, rats were given doses up to 46 mg/kg/day of the carrier and up to 1.23 mg/kg/day of insulin, by nose-only inhalation. No increased incidence of tumors was observed at systemic exposures equivalent to the insulin at a maximum daily AFREZZA dose of 99 mg based on a comparison of relative body surface areas across species.
No increased incidence of tumors was observed in a 26 week carcinogenicity study in transgenic mice (Tg-ras-H2) given doses up to 75 mg/kg/day of carrier and up to 5 mg/kg/day of AFREZZA.
AFREZZA was not genotoxic in Ames bacterial mutagenicity assay and in the chromosome aberration assay, using human peripheral lymphocytes with or without metabolic activation. The carrier alone was not genotoxic in the in vivo mouse micronucleus assay.
In female rats given subcutaneous doses of 10, 30, and 100 mg/kg/day of carrier (vehicle without insulin) beginning 2 weeks prior to mating until gestation day 7, there were no adverse effects on male fertility at doses up to 100 mg/kg/day (a systemic exposure 14-21 times that following the maximum daily AFREZZA dose of 99 mg based on AUC). In female rats there was increased pre- and post-implantation loss at 100 mg/kg/day but not at 30 mg/kg/day (14-21 times higher systemic exposure than the maximum daily AFREZZA dose of 99 mg based on AUC).
14. Clinical Studies
14.1 Overview of Clinical Studies of AFREZZA for Diabetes Mellitus
AFREZZA has been studied in adults with type 1 diabetes in combination with basal insulin. The efficacy of AFREZZA in type 1 diabetes patients was compared to insulin aspart in combination with basal insulin. AFREZZA has been studied in adults with type 2 diabetes in combination with oral antidiabetic drugs. The efficacy of AFREZZA in type 2 diabetes patients was compared to placebo inhalation.
14.2 Type 1 Diabetes
Patients with inadequately controlled type 1 diabetes participated in a 24-week, open-label, active-controlled study to evaluate the glucose lowering effect of mealtime AFREZZA used in combination with a basal insulin. Following a 4-week basal insulin optimization period, 344 patients were randomized to AFREZZA (n=174) or insulin aspart (n=170)administered at each meal of the day. Mealtime insulin doses were titrated to glycemic goals for the first 12 weeks and kept stable for the last 12 weeks of the study. At Week 24, treatment with basal insulin and mealtime AFREZZA provided a mean reduction in HbA1c that met the pre-specified non-inferiority margin of 0.4%. AFREZZA provided less HbA1c reduction than insulin aspart, and the difference was statistically significant. More subjects in the insulin aspart group achieved the HbA1c target of ≤7% (Table 5).
Table 5. Results at Week 24 in an Active-Controlled Study of Mealtime AFREZZA plus Basal Insulin in Adults with Type 1 Diabetes:
| Efficacy Parameter | AFREZZA + Basal Insulin (N=174) | Insulin Aspart + Basal Insulin (N=170) |
|---|---|---|
| HbA1c (%) | ||
| Baseline (adjusted mean *) | 7.94 | 7.92 |
| Change from baseline (adjusted mean*,†) | -0.21 | -0.40 |
| Difference from insulin aspart (adjusted mean*,†) | 0.19 | |
| (95% CI) | (0.02, 0.36) | |
| Percentage of patients achieving HbA1c ≤7%‡ | 13.8 | 27.1 |
| Fasting Plasma Glucose (mg/dL) | ||
| Baseline (adjusted mean*) | 153.9 | 151.6 |
| Change from baseline (adjusted mean*,†) | -25.3 | 10.2 |
| Difference from insulin aspart (adjusted mean *,†) | -35.4 | |
| (95% CI) | (-56.3, -14.6) | |
* Adjusted mean was obtained using a Mixed Model Repeated Measures (MMRM) approach with HbA1c or FPG as the dependent variable and treatment, visit, region, basal insulin stratum, and treatment by visit interaction as fixed factors, and corresponding baseline as a covariate. An autoregression (1) [AR(1)] covariance structure was used.
† Data at 24 weeks were available from 131 (75%) and 150 (88%) subjects randomized to the AFREZZA and insulin aspart groups, respectively.
‡ The percentage was calculated based on the number of patients randomized to the trial.
14.3 Type 2 Diabetes
A total of 479 adult patients with type 2 diabetes inadequately controlled on optimal/maximally tolerated doses of metformin only, or 2 or more oral antidiabetic (OAD) agents participated in a 24-week, double-blind, placebo-controlled study. Following a 6-week run-in period, 353 patients were randomized to AFREZZA (n=177) or an inhaled placebo powder without insulin (n=176). Insulin doses were titrated for the first 12 weeks and kept stable for the last 12 weeks of the study. OADs doses were kept stable. At Week 24, treatment with AFREZZA plus OADs provided a mean reduction in HbA1c that was statistically significantly greater compared to the HbA1c reduction observed in the placebo group (Table 6).
Table 6. Results at Week 24 in a Placebo-Controlled Study of AFREZZA in Adults with Type 2 Diabetes Inadequately Controlled on Oral Antidiabetic Agents:
| Efficacy Parameter | AFREZZA + Oral Anti-Diabetic Agents (N=177) | Placebo + Oral Anti-Diabetic Agents (N=176) |
|---|---|---|
| HbA 1c (%) | ||
| Baseline (adjusted mean*) | 8.25 | 8.27 |
| Change from baseline (adjusted mean*,†) | -0.82 | -0.42 |
| Difference from placebo (adjusted mean*,†) (95% CI) | -0.40 (-0.57, -0.23) | |
| Percentage () of patients achieving HbA 1C ≤7‡ | 32.2 | 15.3 |
| Fasting Plasma Glucose (mg/dL) | ||
| Baseline (adjusted mean*) | 175.9 | 175.2 |
| Change from baseline (adjusted mean*,†) | -11.2 | -3.8 |
| Difference from placebo (adjusted mean*,†) (95% CI) | -7.4 (-18.0, 3.2) | |
* Adjusted mean was obtained using a Mixed Model Repeated Measures (MMRM) approach with HbA1c or FPG as the dependent variable and treatment, visit, region, and treatment by visit interaction as fixed factors, and corresponding baseline as a covariate. An autoregression (1) [AR(1)] covariance structure was used.
† Data at 24 weeks without rescue therapy were available from 139 (79%) and 129 (73%) subjects randomized to the AFREZZA and placebo groups, respectively.
‡ The percentage was calculated based on the number of patients randomized to the trial.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.