AGAMREE Oral suspension Ref.[107213] Active ingredients: Vamorolone
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Santhera Pharmaceuticals (Deutschland) GmbH, Marie-Curie Strasse 8, D-79539 Lörrach, GERMANY, office@santhera.com
5.1. Pharmacodynamic properties
Mechanism of action
Vamorolone is a dissociative corticosteroid that selectively binds to the glucocorticoid receptor, which triggers anti-inflammatory effects via inhibition of NF-kB mediated gene transcripts, but leads to less transcriptional activation of other genes. In addition, vamorolone inhibits the activation of the mineralocorticoid receptor by aldosterone. Due to its specific structure, vamorolone is likely not a substrate for 11β-hydroxysteroid dehydrogenases and is therefore not subject to local tissue amplification. The precise mechanism by which vamorolone exerts its therapeutic effects in patients with DMD is unknown.
Pharmacodynamic effects
Vamorolone produced a dose-dependent decrease in morning cortisol levels in the clinical studies. A dose-dependent increase in haemoglobin, haematocrit values, erythrocytes, leukocyte counts and lymphocyte counts was observed with in clinical studies with vamorolone. No relevant changes in mean neutrophil counts or immature granulocytes were observed. High density lipoprotein (HDL) cholesterol and triglycerides values increased in a dose-dependent manner. There was no relevant effect on glucose metabolism up to 30 months of treatment.
Unlike corticosteroids, vamorolone did not result in a reduction of bone metabolism as measured by bone turnover markers, nor in a significant reduction in lumbar vertebral bone mineralisation parameters by Dual-Energy X-Ray Absorptiometry (DXA) after 48 weeks in the clinical studies. The risk for bone fractures in patients with DMD treated with vamorolone has not been established.
Clinical efficacy and safety
The efficacy of AGAMREE for the treatment of DMD was evaluated in Study 1, a multi-centre, randomised, double-blind, parallel-group, placebo- and active-controlled study of 24 weeks duration followed by a double-blind extension phase. The study population consisted of 121 male paediatric patients 4 to <7 years of age at time of enrolment in the study who were corticosteroid naïve and ambulatory, with a confirmed diagnosis of DMD.
Study 1 randomised 121 patients to one of the following treatments: vamorolone 6 mg/kg/day (n=30), vamorolone 2 mg/kg/day (n=30), active comparator prednisone 0.75 mg/kg/day (n=31), or placebo (n=30). After 24 weeks (Period 1, primary efficacy analysis), patients who had been receiving prednisone or placebo were re-assigned according to an initially defined randomisation scheme to either vamorolone 6 mg/kg/day or 2 mg/kg/day for an additional 20 weeks of treatment (Period 2).
In Study 1, efficacy was evaluated by assessing the change from Baseline to Week 24 in Time to Stand Test (TTSTAND) velocity for vamorolone 6 mg/kg/day compared to placebo. A pre-specified hierarchical analysis of relevant secondary endpoints consisted of change from baseline in TTSTAND velocity for the vamorolone 2 mg/kg/day vs placebo group, change from baseline in 6 Minute Walk Test (6MWT) distance for vamorolone 6 mg/kg/day followed by 2 mg/kg/day vs placebo.
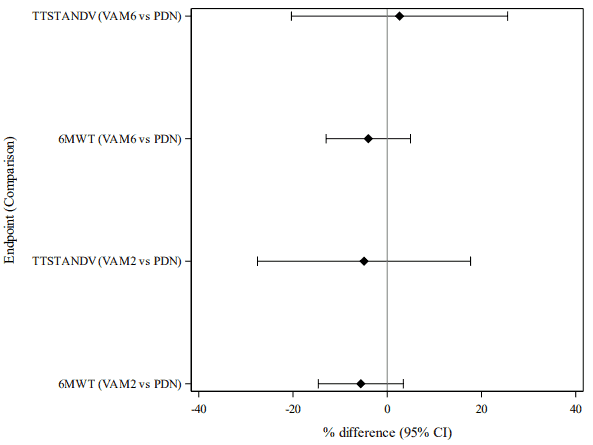
Treatment with vamorolone 6 mg/kg/day and 2 mg/kg/day resulted in a statistically significant improvement in change in TTSTAND velocity and change in 6MWT distance between baseline and Week 24 compared to placebo (see table 2). Study 1 was not designed to maintain the overall Type I error rate for comparisons of each vamorolone group versus prednisone, therefore a global assessment of treatment differences across endpoints, expressed in percentual change from baseline with 95% confidence intervals is presented in Figure 1 for these endpoints.
Table 3. Analysis of change from baseline with vamorolone 6 mg/kg/day or vamorolone 2 mg/kg/day compared to placebo at Week 24 (Study 1):
| TTSTAND velocity (rises/s) / TTSTAND in Seconds (s/rise) | Placebo | Vam 2 mg/kg/day | Vam 6 mg/kg/day | Pred 0.75 mg/kg/day |
| Baseline mean rises/s Baseline mean s/rise | 0.20 5.555 | 0.18 6.07 | 0.19 5.97 | 0.22 4.92 |
| Mean change at 24 weeks Rises /s Improvement in s/rise | -0.012 -0.62 | 0.031 0.31 | 0.046 1.05 | 0.066 1.24 |
| Difference versus placebo* Rises /s s/rise | - | 0.043 (0.007; 0.079) 0.927 (0.042; 1.895) | 0.059 (0.022; 0.095) 1.67 (0.684; 2.658) | not given not given |
| p-value | - | 0.020 | 0.002 | not given |
| 6MWT distance (meters) | Placebo | Vam 2 mg/kg/day | Vam 6 mg/kg/day | Pred 0.75 mg/kg/day |
| Baseline mean (m) | 354.5 | 316.1 | 312.5 | 343.3 |
| Mean change at 24 weeks | -11.4 | +25.0 | +24.6 | +44.1 |
| Difference versus placebo* | - | 36.3 (8.3; 64.4) | 35.9 (8.0; 63.9) | not given |
| p-value | - | 0.011 | 0.012 | not given |
Mean changes and differences are model-based least-squares means (LSM) and mean differences.
Positive numbers indicate improvement as compared with the baseline value.
* Differences in LSM presented with 95% CI
Figure 1. Comparisons between vamorolone and prednisone in timed tests for motor function, analysed as percentual changes from baseline (mITT-1 population):
Test data are standardised by using the percentual change from baseline as the endpoint. The percentile changes are calculated as (value at visit – baseline value) / baseline value x 100%. VAM: Vamorolone, PDN: Prednisone
All the percent-change values from the two endpoints are entered to a single statistical model (MMRM)
For vamorolone 6 mg/kg/day, the improvements in all tested measurements of lower limb function seen at 24 weeks were largely maintained for 48 weeks of treatment, while results across the efficacy outcome measures for the vamorolone 2 mg/kg/day dose were rather inconsistent with declines in relevant functional outcome parameters at Week 48, i.e. TTSTAND velocity and 6MWT, reaching clinically significant differences compared to vamorolone 6 mg/kg/day but only minimal decrease in the NSAA score.
Patients who switched during Study 1 from prednisone 0.75 mg/kg/day in Period 1 to vamorolone 6 mg/kg/day in Period 2 appeared to retain the benefit in terms of these motor function endpoints, while declines were observed in patients that switched to vamorolone 2 mg/kg/day.
At baseline, children in vamorolone groups were smaller in height (median -0.74 SD and -1.04 SD in height z-score for 2 mg/kg/day and 6 mg/kg/day groups, respectively) than children on placebo (-0.54 SD) or prednisone 0.75 mg/kg/day (-0.56 SD). The change in height percentile and height Z-score was similar in children treated with vamorolone or placebo over 24 weeks while they decreased with prednisone. The height percentiles and Z-scores did not decrease with vamorolone over the 48-week study period in Study 1. Switching from prednisone after 24 weeks in Period 1 to vamorolone in Period 2 led to an increase in mean and median height z-score up to Week 48.
5.2. Pharmacokinetic properties
Absorption
Vamorolone is well absorbed and distributes quickly into tissues. After oral administration with food, the median Tmax is about 2 hours (range 0.5 to 5 hours).
Effect of food
Co-administration of vamorolone with a meal reduced Cmax by up to 8% and delayed Tmax by 1 hour, relative to administration under fasting conditions. The overall systemic absorption as measured by AUC was increased by up to 14% when vamorolone was taken with food. The observed differences in absorption do not lead to clinically relevant differences in exposure and therefore vamorolone can be administered either with or without food.
Distribution
The apparent volume of distribution of vamorolone for a DMD patient with a body weight of 20 kg taking vamorolone is 28.5 L based on the population PK analysis. Protein binding is 88.1% in vitro. The blood to plasma ratio is approximately 0.87.
Biotransformation
Vamorolone is metabolised via multiple Phase I and Phase II pathways, such as glucuronidation, hydroxylation, and reduction. The main plasma and urine metabolites are formed through direct glucuronidation as well as hydrogenation with subsequent glucuronidation. The involvement of specific UGT and CYP enzymes in the metabolism of vamorolone has not been conclusively demonstrated.
Elimination
The major route of elimination is by metabolism with subsequent excretion of metabolites into urine and faeces. Vamorolone clearance for a DMD patient with a body weight of 20 kg taking vamorolone is 58 L/h based on the population PK analysis. The terminal elimination half-life of vamorolone in children with DMD is approximately 2 hours.
Approximately 30% of vamorolone dose is excreted in faeces (15.4% unchanged) and 57% of vamorolone dose is excreted in urine as metabolites (<1% unchanged). The major metabolites in urine are glucuronides.
Linearity/non-linearity
The PK are linear and vamorolone exposure increases proportionally with either single or multiple doses. Vamorolone does not accumulate with repeated administration.
Special populations
Hepatic impairment
The effect of moderate hepatic impairment (Child-Pugh class B) of vamorolone was studied in humans. Vamorolone Cmax and AUC0inf values were approximately 1.7- and 2.6-fold higher in subjects with moderate hepatic impairment compared to age, weight and sex matched healthy adults. AGAMREE dose should be reduced in patients with moderate hepatic impairment to 2 mg/kg/day for patients up to 40 kg and to 80 mg for patients with a body weight of 40 kg and above
Based on the available data, the increase in vamorolone exposure is proportional to the severity of hepatic dysfunction. Patients with mild hepatic impairment (Child-Pugh class A) are not expected to have a significant increase in exposure and therefore no dose adjustment is recommended. There is no experience with vamorolone in patients with severe hepatic impairment (Child-Pugh class C) and vamorolone should not be administered to these patients (see section 4.3).
Renal impairment
There is no clinical experience in patients with renal impairment. Vamorolone is not excreted unchanged via the kidney, and increases in exposure due to renal impairment are considered unlikely.
Transporter-mediated interactions
Vamorolone is not an inhibitor of P-gp, BCRP, OATP1B1, OATP1B3, OCT2, OAT1, MATE1, or BSEP. Vamorolone shows weak inhibition of OAT3 and MATE2-K transporters in vitro. Vamorolone is not a substrate of P-gp, BCRP, OATP1A2, OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1, MATE2-K or BSEP.
Paediatric population
At steady state, the geometric mean Cmax and the geometric mean AUC of vamorolone in children (ages 4-7 years) were estimated by Population PK to 1200 ng/ml (CV%=26.8) and 3650 ng/ml.h respectively after administration of 6 mg/kg vamorolone daily.
5.3. Preclinical safety data
Repeat-dose toxicity
Repeated vamorolone administration resulted in transient increases of triglycerides and cholesterol as well as liver enzymes in mice and dogs. Focal hepatic inflammation/necrosis observed in both species might have developed secondary to the hepatocellular hypertrophy and vacuolation containing glycogen and lipid accumulations that likely reflect the stimulation of gluconeogenesis.
Long-term vamorolone dosing also caused adrenal cortex atrophy in mice and dogs, which are ascribable to the known suppression of the hypothalamic-pituitary-adrenal axis by glucocorticoid agents.
The primary anti-inflammatory activity of vamorolone further accounted for mild to moderate lymphocyte depletion in spleen, thymus and lymph nodes of both species. The adverse liver and adrenal gland findings and the lymphoid changes in mice and dogs developed with no safety margins to the MRHD based on AUC.
Genotoxicity and carcinogenicity
Vamorolone did not exert any genotoxic potential in the standard test battery. Carcinogenicity studies have not been conducted with vamorolone, but the absence of pre-neoplastic lesions in long-term toxicity studies and experience with other glucocorticoid agents do not suggest a particular carcinogenic hazard.
Reproductive and developmental toxicity
No standard reproductive and developmental toxicity studies have been performed. Vamorolone did not adversely affect the development of sperm and reproductive tissues in the chronic toxicity study in mice. Following chronic dosing in dogs, incompletely reversible spermatocyte/spermatid degenerations were observed in testes leading to oligospermia and germ cell debris in epididymides. Furthermore, the prostate glands were reduced and contained less secretory product.
In female animals, long-term repeated dosing in dogs additionally resulted in partially reversible bilateral absence of corpora lutea in the ovaries. The inhibition of male and female fertility is attributable to the known interference of long-term glucocorticoid treatment with the hypothalamuspituitary-gonadal axis and developed without AUC-based safety margin to humans at the MRHD.
Juvenile toxicity
The main target organs of vamorolone in male and female juvenile mice overlap with those of adult mice such as adrenal cortical atrophy and vamorolone-related adverse hepatocellular degeneration/necrosis.
Vamorolone-related effects exclusively observed in juvenile mice were non-adverse tibia and body lengths reductions in male and female animals and were attributed to the induction of slower growths. In addition, acinar cell hypertrophy of mandibular salivary glands were detected in female animals. Whereas growth retardation is a well known effect associated with glucocorticoid treatment of children, the relevance of the salivary gland findings for children is unknown. At the no observed adverse effect level (NOAEL) for general toxicity in male and female juvenile mice, no safety margin with respect to human exposure at the MRHD exists.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.