ALTUVOCT Powder and solvent for solution for injection Ref.[111575] Active ingredients: Efanesoctocog alfa
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Swedish Orphan Biovitrum AB (publ), SE-112 76 Stockholm, Sweden
4.1. Therapeutic indications
Treatment and prophylaxis of bleeding in patients with haemophilia A (congenital factor VIII deficiency).
ALTUVOCT can be used for all age groups.
4.2. Posology and method of administration
Treatment should be under the supervision of a physician experienced in the treatment of haemophilia.
After proper training in the correct injection technique (see section 6.6 and package leaflet), a patient may self-inject ALTUVOCT, or the patient’s caregiver may administer it, if their physician determines that it is appropriate.
Treatment monitoring
Individual patients may vary in their response to factor VIII, demonstrating different half-lives and recoveries. Dose based on bodyweight may require adjustment in underweight or overweight patients. Monitoring of factor VIII levels for the purpose of dose adjustment is usually not necessary during routine prophylaxis. In case of major surgery or life-threatening bleeds, determination of factor VIII levels is required to guide the dose and frequency of repeated injections.
When using an in vitro thromboplastin-time (aPTT)-based one-stage clotting assay for determining factor VIII activity in patients' blood samples, plasma factor VIII activity results can be significantly affected by both the type of aPTT reagent and the reference standard used in the assay. Also there can be significant discrepancies between assay results obtained by aPTT-based one-stage clotting assay and the chromogenic assay according to Ph. Eur. This is of importance particularly when changing the laboratory and/or reagents used in the assay.
It is recommended to use a validated one-stage clotting assay to determine plasma factor VIII activity of ALTUVOCT. Throughout the clinical development an Actin-FSL-based one-stage clotting assay was used.
According to the findings of a comparative analysis of clinical study samples, results obtained using a chromogenic assay should be divided by 2.5 to approximate the patient’s factor VIII activity (see section 4.4). In addition, a field study comparing different aPTT reagents indicated approximately 2.5-fold higher factor VIII activity levels when using Actin-FS instead of Actin-FSL in the one-stage clotting assay and approximately 30% lower results when using SynthASil.
Posology
The dose and duration of the substitution therapy depend on the severity of the factor VIII deficiency, on the location and extent of the bleeding and on the patient’s clinical condition.
The number of units of factor VIII administered is expressed in International Units (IU), which are related to the current WHO concentrate standard for factor VIII products. Factor VIII activity in plasma is expressed either as a percentage (relative to normal human plasma) or preferably in International Units (relative to an International Standard for factor VIII in plasma).
One IU of factor VIII activity is equivalent to that quantity of factor VIII in one mL of normal human plasma.
For the dose of 50 IU factor VIII per kg body weight, t he expected in vivo plasma recovery in factor VIII level expressed as IU/dL (or % of normal) is estimated using the following formula:
Estimated increment of factor VIII (IU/dL or % of normal) = 50 IU/kg x 2 (IU/dL per IU/kg)
On demand treatment
ALTUVOCT dosing for the on-demand treatment, control of bleeding episodes and perioperative management is provided in Table 1.
Table 1. Guide to ALTUVOCT dosing for treatment of bleeding episodes and surgery:
| Degree of haemorrhage/Type of surgical procedure | Recommended dose | Additional information |
|---|---|---|
| Haemorrhage | ||
| Single dose of 50 IU/kg | For minor and moderate bleeding episodes occurring within 2 to 3 days after a prophylactic dose, a lower dose of 30 IU/kg dose may be used. An additional dose of 30 or 50 IU/kg after 2 to 3 days may be considered. | |
| More extensive haemarthrosis, muscle bleeding or haematoma | Single dose of 50 IU/kg | Additional doses of 30 or 50 IU/kg every 2 to 3 days may be considered until bleeding is resolved. |
| Life threatening haemorrhages | Single dose of 50 IU/kg | Additional doses of 30 or 50 IU/kg every 2 to 3 days may be administered until the threat is resolved. |
| Surgery | ||
| Minor surgery including tooth extraction | Single dose of 50 IU/kg | An additional dose of 30 or 50 IU/kg after 2 to 3 days may be considered. |
| Major surgery | Single dose of 50 IU/kg | Additional doses of 30 or 50 IU/kg every 2 to 3 days may be administered as clinically needed until adequate wound healing is achieved. |
For resumption of prophylaxis (if applicable) after treatment of a bleed, it is recommended to allow an interval of at least 72 hours between the last 50 IU/kg dose for treatment of a bleed and resuming prophylaxis dosing. Thereafter, prophylaxis can be continued as usual on the patient’s regular dosing schedule.
Prophylaxis
The recommended dosing for routine prophylaxis for adults and children is 50 IU/kg of ALTUVOCT administered once weekly.
Special populations
Elderly
There is limited experience in patients ≥65 years. The dosing recommendations are the same as for patients <65 years.
Paediatric population
The dosing recommendations are the same as for adults.
Method of administration
Intravenous use.
The entire ALTUVOCT dose should be injected intravenously over 1 to 10 minutes, based on the patient’s comfort level.
For instructions on dilution of the medicinal product before administration, see section 6.6.
4.9. Overdose
No symptoms of overdose with human coagulation factor VIII (rDNA) have been reported.
6.3. Shelf life
Unopened vial:
4 years.
During the shelf-life, the medicinal product may be stored at room temperature (up to 30°C) for a single period not exceeding 6 months. The date that the medicinal product is removed from refrigeration should be recorded on the carton. After storage at room temperature, the medicinal product may not be returned to the refrigerator. Do not use beyond the expiry date printed on the vial or six months after removing the carton from refrigeration, whichever is earlier.
After reconstitution:
The medicinal product should be used immediately after reconstitution. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
6.4. Special precautions for storage
Store in a refrigerator (2°C–8°C).
Do not freeze.
Keep the vial in the outer carton in order to protect from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Each pack of ALTUVOCT 250 IU, 500 IU, 750 IU 1 000 IU, 2 000 IU, 3 000 IU and 4 000 IU powder and solvent for solution for injection contains:
- a glass vial (type I) with powder and chlorobutyl rubber stopper
- a sterile vial adapter for reconstitution
- a pre-filled glass syringe of 3 mL solvent with a bromobutyl rubber plunger stopper
- a plunger rod
- a sterile infusion set
6.6. Special precautions for disposal and other handling
ALTUVOCT is to be administered intravenously after reconstitution of the powder with the solvent supplied in the syringe. The vial should be gently swirled until all of the powder is dissolved. After reconstitution the solution should be clear and colourless to slightly opalescent. Do not use solutions that are cloudy or have deposits.
Always use an aseptic technique.
Additional information on reconstitution
ALTUVOCT is administered by intravenous injection after dissolving the powder for injection with the solvent supplied in the pre-filled syringe. ALTUVOCT pack contains:
You will also need sterile alcohol swabs (F). This device is not included in the ALTUVOCT package.
To draw up the solution from multiple vials into a single syringe you may use a separate large syringe (G). If a large syringe is not available, follow steps 6 to 8 to administer the solution from each syringe.
ALTUVOCT should not be mixed with other solutions for injection or infusion.
Wash your hands before opening the pack.
Reconstitution:
| 1. Prepare the vial | |
| a. Remove the vial cap Hold the powder vial (A) on a clean flat surface and remove the plastic cap. | 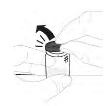 |
| b. Clean vial top Wipe the top of the vial with an alcohol swab. After cleaning, ensure nothing touches the top of the vial. | 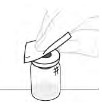 |
| c. Open vial adapter package Peel off the protective paper lid from the vial adapter package (D). Do not touch the vial adapter, or remove it from its package. | 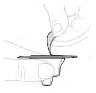 |
| d. Attach vial adapter Place the vial adapter package squarely over the top of the vial. Press down firmly until the adapter snaps into place. The spike will penetrate the vial stopper. | 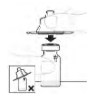 |
| 2. Prepare the syringe | |
| a. Attach plunger rod Insert the plunger rod (C) into the 3 mL syringe (B). Turn the plunger rod clockwise until it is securely attached. |  |
| b. Remove syringe cap Snap off the top part of white 3 mL syringe cap at the perforations and set aside. ⚠ Do not touch the inside of cap or the syringe tip. | 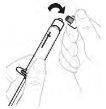 |
| 3. Attach syringe to vial | |
| a. Remove vial adapter package Lift the package away from the vial adapter and dispose. | 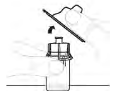 |
| b. Attach syringe to vial adapter Hold the vial adapter at the lower end. Place the syringe tip onto the top of the vial adapter. Turn the syringe clockwise to securely attach. | 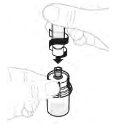 |
| 4. Dissolve the powder and solvent | |
| a. Add solvent to vial Slowly press the plunger rod to inject all the solvent into the vial. | 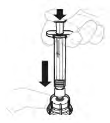 |
| b. Dissolve powder With your thumb on the plunger rod, gently swirl the vial until powder is dissolved. Do not shake. | 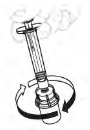 |
| c. Inspect solution Inspect the solution before administration. It should be clear and colourless. Do not use the solution if cloudy or contains visible particles. | |
| 5. If using multiple vials | |
| If your dose requires multiple vials, follow the steps below (5a and 5b) otherwise skip to step 6. | |
| a. Repeat 1 to 4 Repeat steps 1 to 4 with all vials until you have prepared enough solution for your dose. Remove the 3 mL syringes from each vial (see step 6b), leaving the solution in each vial. |  |
| b. Using large syringe (G) For each vial, attach the large syringe (G) to the vial adapter (see step 3b) and perform step 6, to combine the solution from each vial into the large syringe. In case you only need part of an entire vial, use the scale on the syringe to see how much solution you withdraw. | 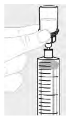 |
| 6. Draw solution into syringe | |
| a. Draw back solution Point the syringe up. Slowly pull the plunger rod to draw all the solution into the syringe. | 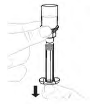 |
| b. Detach syringe Detach the syringe from the vial by holding the vial adapter. Turn the syringe anticlockwise to detach | 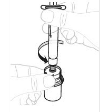 |
It is recommended to use ALTUVOCT immediately after reconstitution (see section 6.3).
Administration:
| 7. Prepare for injection | |
| a. Remove tubing cap Open infusion set (E) packaging (do not use if damaged). Remove the tubing cap. ⚠ Do not touch the exposed end of the tubing set. |  |
| b. Attach syringe Attach prepared syringe to the end of the infusion set tubing by turning the syringe clockwise. | 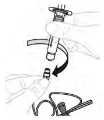 |
| c. Prepare injection site If needed apply a tourniquet. Wipe injection site with an alcohol swab (F). | 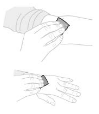 |
| d. Remove air from syringe and tubing Remove air by pointing the syringe up and gently pressing the plunger rod. Do not push the solution through the needle. ⚠Injecting air into the vein can be dangerous. |  |
| 8. Inject solution | |
| a. Insert needle Remove protective needle cover. Insert the needle into a vein and remove the tourniquet if used.  You may use a plaster to hold the plastic wings of the needle in place at the injection site You may use a plaster to hold the plastic wings of the needle in place at the injection siteto prevent movement. | |
| b. Inject solution The prepared solution should be injected intravenously over 1 to 10 minutes, based on the patient’s comfort level. | |
| 9. Dispose safely | |
| a. Remove needle Remove the needle. Fold over the needle protector; it should snap into place. | 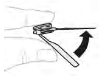 |
| b. Safe disposal Ensure all used components in the provided kit (other than packaging) is safely dispose of in a medical waste container. ⚠ Do not reuse equipment. | |
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.