ANDROGEL 1% Gel Ref.[10748] Active ingredients: Testosterone
Source: FDA, National Drug Code (US) Revision Year: 2020
1. Indications and Usage
AndroGel 1% is indicated for replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone:
- Primary hypogonadism (congenital or acquired): testicular failure due to conditions such as cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter’s syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations, but have gonadotropins in the normal or low range.
Limitations of use:
- Safety and efficacy of AndroGel 1% in men with “age-related hypogonadism” (also referred to as “late-onset hypogonadism”) have not been established.
- Safety and efficacy of AndroGel 1% in males less than 18 years old have not been established [see Use in Specific Populations (8.4)].
- Topical testosterone products may have different doses, strengths or application instructions that may result in different systemic exposure (1, 12.3).
2. Dosage and Administration
Dosage and Administration for AndroGel 1% differs from AndroGel 1.62%. For dosage and administration of AndroGel 1.62% refer to its full prescribing information.
Prior to initiating AndroGel 1%, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum testosterone concentrations are below the normal range.
2.1 Dosing and Dose Adjustment
The recommended starting dose of AndroGel 1% is 50 mg of testosterone (two 25 mg packets or one 50 mg packet), applied topically once daily in the morning to the shoulders and upper arms and/or abdomen area (preferably at the same time every day).
Dose Adjustment
To ensure proper dosing, serum testosterone concentrations should be measured at intervals. If the serum testosterone concentration is below the normal range, the daily AndroGel 1% dose may be increased from 50 mg to 75 mg and from 75 mg to 100 mg for adult males as instructed by the physician. If the serum testosterone concentration exceeds the normal range, the daily AndroGel 1% dose may be decreased. If the serum testosterone concentration consistently exceeds the normal range at a daily dose of 50 mg, AndroGel 1% therapy should be discontinued. In addition, serum testosterone concentrations should be assessed periodically.
The application site and dose of AndroGel 1% are not interchangeable with other topical testosterone products.
2.2 Administration Instructions
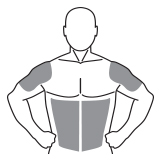
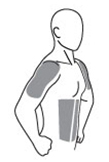
AndroGel 1% should be applied to clean, dry, healthy, intact skin of the right and left upper arms/shoulders and/or right and left abdomen. Area of application should be limited to the area that will be covered by the patient’s short sleeve T-shirt. Do not apply AndroGel 1% to any other part of the body including the genitals, chest, armpits (axillae), knees, or back. AndroGel 1% should be evenly distributed between the right and left upper arms/shoulders or both sides of the abdomen.
The prescribed daily dose of AndroGel 1% should be applied to the right and left upper arms/shoulders and/or right/left abdomen as shown in the shaded areas in the figure below.
After applying the gel, the application site should be allowed to dry prior to dressing. Hands should be washed thoroughly with soap and water after application. Avoid fire, flames or smoking until the gel has dried since alcohol based products, including AndroGel 1%, are flammable.
The patient should be advised to avoid swimming or showering for at least 5 hours after the application of AndroGel 1%.
Packets
The entire contents should be squeezed into the palm of the hand and immediately applied to the application sites. Alternately, patients may squeeze a portion of the gel from the packet into the palm of the hand and apply to application sites. Repeat until entire contents have been applied.
Strict adherence to the following precautions is advised in order to minimize the potential for secondary exposure to testosterone from AndroGel 1%-treated skin:
- Children and women should avoid contact with unwashed or unclothed application site(s) of men using AndroGel 1%.
- Patients should wash hands with soap and water immediately after application of AndroGel 1%.
- Patients should cover the application site(s) with clothing (e.g., a T-shirt) after the gel has dried.
- Prior to situation in which direct skin-to-skin contact is anticipated, patients should wash the application site thoroughly with soap and water to remove any testosterone residue.
- In the event that unwashed or unclothed skin to which AndroGel 1% has been applied comes in direct contact with the skin of another person, the general area of contact on the other person should be washed with soap and water as soon as possible.
10. Overdosage
There is one report of acute overdosage with use of an approved injectable testosterone product: this subject had serum testosterone concentrations of up to 11,400 ng/dL with a cerebrovascular accident.
Treatment of overdosage would consist of discontinuation of AndroGel 1%, washing the application site with soap and water, and appropriate symptomatic and supportive care.
16.2. Storage and Handling
Storage: Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Disposal: Used AndroGel 1% packets should be discarded in household trash in a manner that prevents accidental application or ingestion by children or pets.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.