AWIQLI Solution for injection Ref.[110293] Active ingredients: Insulin icodec
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Novo Nordisk A/S, Novo Alle 1, DK-2880 Bagsvaerd, Denmark
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes, insulins and analogues for injection, long-acting
ATC code: A10AE07
Mechanism of action
A slow and steady glucose-lowering effect of insulin icodec is driven by albumin binding as well as reduced insulin receptor binding and clearance. The extended half-life of insulin icodec reflects a depot of insulin icodec in the circulation and in the interstitial compartment, from which insulin icodec is slowly and continuously released and binds specifically to the insulin receptor. When insulin icodec binds to the human insulin receptor it results in the same pharmacological effects as human insulin.
The primary action of insulin, including insulin icodec, is to regulate glucose metabolism. Insulin and its analogues lower blood glucose by activating specific insulin receptors to stimulate peripheral glucose uptake, especially by skeletal muscle and fat as well as to inhibit hepatic glucose production. Insulin also inhibits lipolysis and proteolysis and enhances protein synthesis.
Pharmacodynamic effects
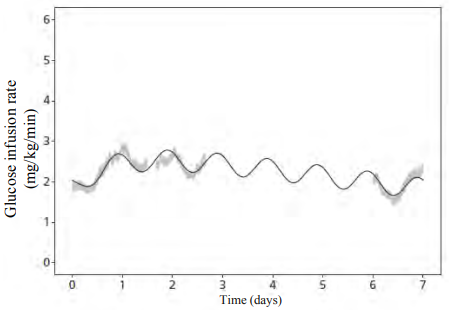
The steady-state pharmacodynamic properties of insulin icodec were investigated in a trial with type 2 diabetes patients. The partial pharmacodynamic properties of insulin icodec were measured in 3 euglycaemic clamps (6.7 mmol/L) during steady state covering 3.5 of the 7 days dosing interval. Glucose infusion rate (GIR) profiles for all three clamps are shown in together with the model-derived data, suggesting the duration of the glucose-lowering effect to cover a full week (Figure 1).
Figure 1. Full-week glucose infusion rate profile of insulin icodec at steady-state in type 2 diabetes:
Notes: Shaded areas are standard error of the mean of individual glucose infusion rate (GIR) profiles (pooled across three steady-state weeks). Line is mean of individual model-predicted GIR profiles (for one steady-state week).
Based on data where insulin icodec was injected at 20:00 (corresponding to day 0).
Clinical steady state was reached after 2-4 weeks when initiating insulin icodec without a one-time additional dose and after 2-3 weeks when initiating insulin icodec with a one-time additional dose of 50% with the first dose.
Clinical efficacy and safety
The safety and efficacy of insulin icodec were evaluated in five multinational, randomised, activecontrolled, open-label or blinded, parallel-group phase 3 clinical trials of 26 or 52 weeks duration (ONWARDS 1-4 and 6). The trials exposed 1 628 patients to insulin icodec (1 338 in type 2 diabetes mellitus and 290 in type 1 diabetes mellitus). A treat-to-target approach was followed in the trials. The glycaemic target was fasting pre-breakfast self-measured plasma glucose (SMPG) values of 4.4-7.2 mmol/L. Based on the last 3 pre-breakfast SMPG values, the insulin icodec dose was kept stable or adjusted up or down according to trial schedule (weekly or every other week).
The safety and efficacy of insulin icodec were evaluated in insulin-naïve type 2 diabetes mellitus patients (ONWARDS 1 and 3), in type 2 diabetes mellitus patients previously treated with basal insulin (ONWARDS 2), in type 2 diabetes mellitus patients previously treated with basal-bolus regimen (ONWARDS 4) and in patients with type 1 diabetes mellitus (ONWARDS 6). The primary objective for the phase 3 trials was to demonstrate the effect on glycaemic control of once-weekly insulin icodec compared to a daily basal insulin (insulin degludec or insulin glargine) in the specific diabetes population investigated. This included comparison of the change in HbA1c from baseline to end of treatment with the comparator to confirm non-inferiority. Patients with severe renal impairment (eGFR <30 mL/min/1.73 m²) were excluded from ONWARDS 1-4 and 6.
Patients with type 2 diabetes mellitus
In a 52-week open-label trial with a 26-week extension phase (ONWARDS 1), 984 insulin naïve type 2 diabetes patients were randomised to insulin icodec and insulin glargine (100 units/mL). At baseline, the patients had a mean duration of diabetes of 11.5 years, mean HbA1c of 69 mmol/mol (8.5%), mean fasting plasma glucose (FPG) of 10.3 mmol/L and a mean BMI of 30.1 kg/m² (Table 3).
In a 26-week double blind trial (ONWARDS 3), 588 insulin naïve type 2 diabetes patients were randomised to insulin icodec and insulin degludec (100 units/mL). At baseline, the patients had a mean duration of diabetes of 11.3 years, mean HbA1c of 69 mmol/mol (8.5%), mean FPG of 10.1 mmol/L and a mean BMI of 29.6 kg/m². The trial was stratified according to region and treatment with sulfonylurea or glinides (Table 3).
In a 26-week open-label trial (ONWARDS 2), 526 basal insulin treated type 2 diabetes patients were randomised to insulin icodec and insulin degludec (100 units/mL). At baseline, the patients had a mean duration of diabetes of 16.7 years, mean HbA1c of 65 mmol/mol (8.1%), mean FPG of 8.4 mmol/L and a mean BMI of 29.3 kg/m² (Table 4).
In a 26-week open-label trial (ONWARDS 4), 582 basal-bolus treated type 2 diabetes patients were randomised to insulin icodec and insulin glargine (100 units/mL). At baseline, the patients had a mean duration of diabetes of 17.1 years, mean HbA1c of 67 mmol/mol (8.3%), mean FPG of 9.4 mmol/L and a mean BMI of 30.3 kg/m² (Table 5).
The trials with type 2 diabetes mellitus patients allowed the maintenance of current non-insulin antidiabetic treatment at the same dose level, except for glinides or sulfonylureas. To minimise the risk of hypoglycaemia, treatment with glinides or sulfonylureas was to be discontinued (ONWARDS 1-2 and 4) or reduced by approximately 50% at randomisation (ONWARDS 3).
Table 3. Results from double-blinded (26 weeks) and open-label (52 weeks) clinical trials in adults with type 2 diabetes mellitus (insulin naïve) – ONWARDS 3 and ONWARDS 1:
| 26 weeks of treatment – ONWARDS 3 | 52 weeks of treatment – ONWARDS 1 | |||
| Insulin icodec | Insulin degludec | Insulin icodec | Insulin glargine 100 units/mL | |
| N (Full Analysis Set) | 294 | 294 | 492 | 492 |
| HbA1c (mmol/mol) | ||||
| Baseline | 69.96 | 69.23 | 69.44 | 68.79 |
| End of trial* | 52.42 | 54.71 | 52.21 | 54.34 |
| Change from baseline* | -17.18 | -14.88 | -16.91 | -14.78 |
| Estimated difference | -2.30 [-3.73; -0.87]a | -2.13 [-3.93; -0.32]a | ||
| HbA1c (%) | ||||
| Baseline | 8.55 | 8.48 | 8.50 | 8.44 |
| End of trial* | 6.95 | 7.16 | 6.93 | 7.12 |
| Change from baseline* | -1.57 | -1.36 | -1.55 | -1.35 |
| Estimated difference | -0.21 [-0.34; -0.08]a | -0.19 [-0.36; -0.03]a | ||
| Patients (%) achieving HbA1c | ||||
| <7% without level 2 or 3 hypoglycaemia* | 52.13 | 39.86 | 52.56 | 42.58 |
| Estimated odds ratio | 1.64 [1.16; 2.33]b,c | 1.49 [1.15; 1.94]b,c | ||
| Fasting plasma glucose (mmol/L) | ||||
| Baseline | 10.37 | 9.78 | 10.28 | 10.31 |
| End of trial* | 7.06 | 7.08 | 6.95 | 6.96 |
| Change from baseline* | -3.01 | -2.99 | -3.35 | -3.33 |
| Estimated difference | -0.02 [-0.34; 0.29]b | -0.01 [-0.27; 0.24]b | ||
| Time in Range (3.9-10.0 mmol/L) (%) | ||||
| Weeks 48-52 | N/A | 71.94 | 66.90 | |
| Estimated difference | N/A | 4.27 [1.92; 6.62]; p<0.001a,d | ||
| Rate of hypoglycaemia per PYE (percentage of patients) | ||||
| Level 2 | 0.31 (8.9) | 0.13 (5.8) | 0.29 (9.8) | 0.15 (10.0) |
| Estimated rate ratio | 2.09 [0.99; 4.41]b | 1.67 [0.99; 2.84]b | ||
| Level 3 | 0 (0) | 0.01 (0.7) | <0.01 (0.2) | 0 (0.6) |
| Level 2 or level 3 | 0.31 (8.9) | 0.15 (6.1) | 0.30 (9.8) | 0.16 (10.6) |
| Estimated rate ratio | 1.82 [0.87; 3.80]b | 1.64 [0.98; 2.75]b | ||
PYE = patient years of exposure
The 95% confidence interval is stated in "[]"
* Least Squares (LS) mean
a p<0.05 for superiority, adjusted for multiplicity
b no correction for multiplicity
c higher odds of achieving HbA1c target without level 3 or level 2 hypoglycaemia in the prior 12 weeks in patients treated with insulin icodec
d 4.27% corresponds to approximately 61 minutes more spent within range per day.
Table 4. Results from open-label clinical trial in adults with type 2 diabetes mellitus (patients previously treated with basal insulin only) – ONWARDS 2:
| 26 weeks of treatment | ||
| Insulin icodec | Insulin degludec | |
| N (Full Analysis Set) | 263 | 263 |
| HbA1c (mmol/mol) | ||
| Baseline | 65.76 | 65.02 |
| End of trial* | 55.19 | 57.64 |
| Change from baseline* | -10.20 | -7.75 |
| Estimated difference | -2.45 [-4.05; -0.84]a | |
| HbA1c (%) | ||
| Baseline | 8.17 | 8.10 |
| End of trial* | 7.20 | 7.42 |
| Change from baseline* | -0.93 | -0.71 |
| Estimated difference | -0.22 [-0.37; -0.08]a | |
| Patients (%) achieving HbA1c | ||
| <7% without level 2 or 3 hypoglycaemia* | 36.73 | 26.79 |
| Estimated odds ratio | 1.59 [1.07; 2.36]b,c | |
| Fasting plasma glucose (mmol/L) | ||
| Baseline | 8.45 | 8.36 |
| End of trial* | 6.83 | 6.79 |
| Change from baseline* | -1.58 | -1.62 |
| Estimated difference | 0.04 [-0.28; 0.36]b | |
| Time in Range (3.9-10.0 mmol/L) (%) | ||
| Weeks 22-26 | 63.13 | 59.50 |
| Estimated difference | 2.41 [-0.84; 5.65]b,d | |
| Rate of hypoglycaemia per PYE (percentage of patients) | ||
| Level 2 | 0.73 (14.1) | 0.27 (7.2) |
| Estimated rate ratio | 1.98 [0.95; 4.12]b | |
| Level 3 | 0 (0) | 0.01 (0.4) |
| Level 2 or level 3 | 0.73 (14.1) | 0.27 (7.2) |
| Estimated rate ratio | 1.93 [0.93; 4.02]b | |
PYE = patient years of exposure
The 95% confidence interval is stated in "[]"
* Least Squares (LS) mean
a p<0.05 for superiority, adjusted for multiplicity
b no correction for multiplicity
c higher odds of achieving HbA1c target without level 3 or level 2 hypoglycaemia in the prior 12 weeks in patients treated with insulin icodec
d 2.41% corresponds to approximately 35 minutes more spent within range per day.
Table 5. Results from open-label clinical trial in adults with type 2 diabetes mellitus (patients previously treated with basal-bolus regimen) – ONWARDS 4:
| 26 weeks of treatment | ||
| Insulin icodec | Insulin glargine 100 units/mL | |
| N (Full Analysis Set) | 291 | 291 |
| HbA1c (mmol/mol) | ||
| Baseline | 67.11 | 67.35 |
| End of trial* | 54.58 | 54.35 |
| Change from baseline* | -12.65 | -12.88 |
| Estimated difference | 0.22 [-1.20; 1.65]a | |
| HbA1c (%) | ||
| Baseline | 8.29 | 8.31 |
| End of trial* | 7.14 | 7.12 |
| Change from baseline* | -1.16 | -1.18 |
| Estimated difference | 0.02 [-0.11; 0.15]a | |
| Patients (%) achieving HbA1c | ||
| <7% without level 2 or 3 hypoglycaemic episodes* | 26.48 | 25.24 |
| Estimated odds ratio | 1.07 [0.73; 1.55]b | |
| Fasting plasma glucose (mmol/L) | ||
| Baseline | 9.24 | 9.60 |
| End of trial* | 7.67 | 7.81 |
| Change from baseline* | -1.75 | -1.61 |
| Estimated difference | -0.14 [-0.59; 0.31]b | |
| Time in Range (3.9-10.0 mmol/L) (%) | ||
| Weeks 22-26 | 66.88 | 66.44 |
| Estimated difference | 0.29 [-2.52; 3.09]b,c | |
| Rate of hypoglycaemia per PYE (percentage of patients) | ||
| Level 2 | 5.60 (50.9) | 5.61 (55.0) |
| Estimated rate ratio | 0.99 [0.73; 1.34]b | |
| Level 3 | 0.04 (1.4) | 0.02 (0.7) |
| Estimated rate ratio | 2.19 [0.20; 24.44]b | |
| Level 2 or level 3 | 5.64 (51.5) | 5.62 (55.7) |
| Estimated rate ratio | 0.99 [0.73; 1.33]b | |
PYE = patient years of exposure
The 95% confidence interval is stated in "[]"
* Least Squares (LS) mean
a p<0.05 for non-inferiority, adjusted for multiplicity. The non-inferiority margin of 0.3% was chosen for this endpoint
b no correction for multiplicity
c 0.29% corresponds to approximately 4 minutes more spent within range per day.
Patients with type 1 diabetes mellitus
In a 26-week open-label trial with a 26-week extension phase (ONWARDS 6), 582 basal-bolus treated patients with type 1 diabetes were randomised to insulin icodec and insulin degludec (100 units/mL). At baseline, the patients had a mean duration of diabetes of 19.5 years, mean HbA1c of 60 mmol/mol (7.6%), mean FPG of 9.8 mmol/L and a mean BMI of 26.5 kg/m². The trial was stratified by pre-trial basal insulin treatment (either twice daily/insulin glargine 300 units/mL or once daily) and HbA1c (either <8% or ≥8%) at screening (Table 6).
Table 6. Results from open-label clinical trial in adults with type 1 diabetes mellitus – ONWARDS 6:
| 26 weeks of treatment | ||
| Insulin icodec | Insulin degludec | |
| N (Full Analysis Set) | 290 | 292 |
| HbA1c (mmol/mol) | ||
| Baseline | 59.46 | 59.95 |
| End of trial* | 54.62 | 54.09 |
| Change from baseline* | -5.08 | -5.61 |
| Estimated difference | 0.53 [-1.46; 2.51]a | |
| HbA1c (%) | ||
| Baseline | 7.59 | 7.63 |
| End of trial* | 7.15 | 7.10 |
| Change from baseline* | -0.47 | -0.51 |
| Estimated difference | 0.05 [-0.13; 0.23]a | |
| Patients (%) achieving HbA1c | ||
| <7% without level 2 or 3 hypoglycaemic episodes* | 9.55 | 16.74 |
| Estimated odds ratio | 0.52 [0.33; 0.85]b,c | |
| Fasting plasma glucose (mmol/L) | ||
| Baseline | 9.94 | 9.56 |
| End of trial* | 8.91 | 7.88 |
| Change from baseline* | -0.84 | -1.87 |
| Estimated difference | 1.03 [0.48; 1.59]b | |
| Time in Range (3.9-10.0 mmol/L) (%)** | ||
| Weeks 22-26 | 59.10 | 60.85 |
| Estimated difference | -2.00 [-4.38; 0.38]b,d | |
| Rate of hypoglycaemia per PYE (percentage of patients) | ||
| Level 2 | 19.60 (84.8) | 10.26 (76.4) |
| Estimated rate ratio | 1.88 [1.53; 2.32]b | |
| Level 3 | 0.33 (3.1) | 0.12 (3.1) |
| Estimated rate ratio | 2.08 [0.39; 10.96]b | |
| Level 2 or level 3 | 19.93 (85.2) | 10.37 (76.4) |
| Estimated rate ratio | 1.89 [1.54; 2.33]b | |
PYE = patient years of exposure
The 95% confidence interval is stated in "[]"
* Least Squares (LS) mean
** unblinded CGM data was captured from a trial in patients with type 1 diabetes mellitus
a p<0.05 for non-inferiority, adjusted for multiplicity. The non-inferiority margin of 0.3% was chosen for this endpoint
b no correction for multiplicity
c higher odds of achieving HbA1c target without level 3 or level 2 hypoglycaemia in the prior 12 weeks in patients treated with insulin degludec
d -2.00% corresponds to approximately 29 minutes less spent within range per day.
Extension data for ONWARDS 6
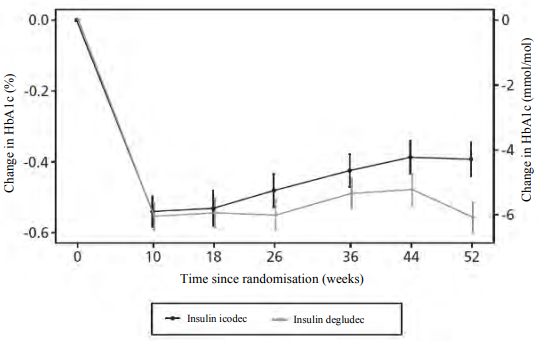
In the complete ONWARDS 6 trial, including the 26-week extension phase, in T1DM patients, the reduction in HbA1c from baseline for insulin icodec vs insulin degludec was -0.37% vs -0.54% (Least Squares [LS] mean; estimated treatment difference 0.17 [0.02;0.31]).
Figure 2. HbA1c by treatment week in ONWARDS 6 – change from baseline up to week 52:
Notes: Observed data including data obtained after premature treatment discontinuation. Full analysis set.
Legend: Mean (symbol) ± standard error to mean (error bars).
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of trials with Awiqli in all subsets of the paediatric population (0 to 18 years) for both type 1 and type 2 diabetes mellitus (see section 4.2 for information on paediatric use).
Immunogenicity
In patients with type 2 diabetes, treatment with insulin icodec induced development of anti-drug antibodies (ADA) in 77%-82% of previously insulin-naïve patients (ONWARDS 3 and trial 4383), in 54% of patients previously treated with daily basal insulin (ONWARDS 2) and in 41% of patients previously treated with daily basal-bolus insulin (ONWARDS 4). In the type 1 diabetes population (ONWARDS 6), treatment with insulin icodec induced development of ADA in 33%. ADA titres were increased in 37% of patients with type 1 diabetes that were ADA positive at baseline. Most of the icodec antibody positive patients, in both the type 1 and type 2 diabetes populations, had also crossreacting antibodies towards human insulin. Overall, the titres of anti-insulin icodec antibodies did not affect the measured clinical efficacy or safety parameters. See also sections 4.4 and 5.2.
Special populations
Improvement in HbA1c was not affected by sex, ethnicity, age, diabetes duration (<10 years and ≥10 years), HbA1c value at baseline (<8% or ≥8%) or baseline body mass index (BMI).
5.2. Pharmacokinetic properties
Overall, pharmacokinetic (PK) properties were similar between groups assessed by population-PK analysis in confirmatory trials, with a trend towards higher exposure with higher anti-drug antibodies (ADA) titres. The effect is not considered clinically relevant as the relative exposure (Cavg) was inside the 0.8-1.25 interval when compared to ADA-negative subjects. Overall ADA prevalence was 70-82%. See section 5.1.
Absorption
Insulin icodec is a basal insulin that binds reversibly to albumin, resulting in a slow release of insulin icodec from the essentially inactive depot in circulation and interstitial compartment.
After subcutaneous injection, clinical steady state was reached after 2-4 weeks when initiating insulin icodec without a one-time additional dose and after 2-3 weeks when initiating insulin icodec with a one-time additional dose of 50% with the first dose.
After subcutaneous injection of insulin icodec, the week-to-week intra-subject variability in total exposure is considered low (coefficient of variation for insulin icodec at steady state was 5.90% in type 2 diabetes patients).
Distribution
The affinity of insulin icodec to serum albumin corresponds to a plasma protein binding of >99% in human plasma. No clinically relevant differences in pharmacokinetics properties of insulin icodec are seen across serum albumin levels.
The results of the in vitro protein binding studies demonstrate that there is no clinically relevant interaction between insulin icodec and fatty acids or other protein-bound medicinal products.
Biotransformation
Degradation of insulin icodec is similar to that of human insulin; all metabolites formed are inactive.
Elimination
The half-life after subcutaneous administration is approximately one week independent of dose.
Linearity
Dose proportionality in total exposure is observed after subcutaneous administration within the therapeutic dose range.
Sex, elderly, renal and hepatic impairment
Overall, the pharmacokinetic properties of insulin icodec were preserved and there was no clinically relevant difference in exposure between female and male subjects, between elderly and younger adult subjects (range of studied age of 18-86 years old), or between healthy subjects and subjects with renal or hepatic impairment.
5.3. Preclinical safety data
The ratio of mitogenic relative to metabolic potency for insulin icodec is comparable to that of human insulin.
Non-clinical data reveal no special safety concerns for humans based on studies of safety pharmacology, repeated dose toxicity, and toxicity to reproduction.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.