BAVENCIO Concentrate for solution for infusion Ref.[6315] Active ingredients: Avelumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Merck Europe B.V., Gustav Mahlerplein 102, 1082 MA Amsterdam, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Other antineoplastic agents, monoclonal antibodies
ATC code: L01FF04
Mechanism of action
Avelumab is a human immunoglobulin G1 (IgG1) monoclonal antibody directed against programmed death ligand 1 (PD-L1). Avelumab binds PD-L1 and blocks the interaction between PD-L1 and the programmed death 1 (PD-1) and B7.1 receptors. This removes the suppressive effects of PD-L1 on cytotoxic CD8+ T-cells, resulting in the restoration of anti-tumour T-cell responses. Avelumab has also shown to induce natural killer (NK) cell-mediated direct tumour cell lysis via antibody-dependent cell-mediated cytotoxicity (ADCC).
Clinical efficacy and safety
Merkel cell carcinoma (study EMR100070-003)
The efficacy and safety of avelumab was investigated in the single arm, multi-centre study EMR100070-003 with two parts. Part A was conducted in patients with histologically confirmed metastatic MCC, whose disease had progressed on or after chemotherapy administered for distant metastatic disease, with a life expectancy of more than 3 months. Part B included patients with histologically confirmed metastatic MCC who were treatment-naïve to systemic therapy in the metastatic setting.
Patients with active or a history of central nervous system (CNS) metastasis; active or a history of autoimmune disease; a history of other malignancies within the last 5 years; organ transplant; conditions requiring therapeutic immune suppression or active infection with HIV, or hepatitis B or C were excluded.
Patients received avelumab at a dose of 10 mg/kg every 2 weeks until disease progression or unacceptable toxicity. Patients with radiological disease progression not associated with significant clinical deterioration, defined as no new or worsening symptoms, no change in performance status for greater than two weeks, and no need for salvage therapy could continue treatment.
Tumour response assessments were performed every 6 weeks, as assessed by an Independent Endpoint Review Committee (IERC) using Response Evaluation Criteria in Solid Tumours (RECIST) v1.1.
Study 003 Part A – previously-treated patients
The major efficacy outcome measure was confirmed best overall response (BOR); secondary efficacy outcome measures included duration of response (DOR), progression-free survival (PFS), and overall survival (OS).
An efficacy analysis was conducted in all 88 patients after a minimum follow-up of 36 months. Patients received a median of 7 doses of avelumab (range: 1 dose to 95 doses), and the median duration of treatment was 17 weeks (range: 2 weeks to 208 weeks).
Of the 88 patients, 65 (74%) were male, the median age was 73 years (range 33 years to 88 years), 81 (92%) patients were Caucasian, and 49 (56%) patients and 39 (44%) patients with an Eastern Cooperative Oncology Group (ECOG) performance status 0 and 1, respectively.
Overall, 52 (59%) patients were reported to have had 1 prior anti-cancer therapy for MCC, 26 (30%) with 2 prior therapies, and 10 (11%) with 3 or more prior therapies. Forty-seven (53%) of the patients had visceral metastases.
Table 4 summarises efficacy endpoints in patients receiving avelumab at the recommended dose for study EMR100070-003, Part A with a minimum follow-up of 36 months. Overall survival was evaluated in an analysis with a minimum follow-up of 44 months. The median OS was 12.6 months (95% CI 7.5, 17.1).
Table 4. Response to avelumab 10 mg/kg every 2 weeks in patients with metastatic MCC in study EMR100070-003 (Part A)*:
| Efficacy endpoints (Part A) (per RECIST v1.1, IERC) | Results (N=88) |
|---|---|
| Objective response rate (ORR) | |
| Response rate, CR+PR** n () (95 CI) | 29 (33.0%) (23.3, 43.8) |
| Confirmed best overall response (BOR) | |
| Complete response (CR)** n () Partial response (PR)** n () | 10 (11.4%) 19 (21.6%) |
| Duration of response (DOR)a | |
| Median, months (95% CI) Minimum, maximum (months) ≥6 months by K-M, (95% CI) ≥12 months by K-M, (95% CI) ≥24 months by K-M, (95% CI) ≥36 months by K-M, (95% CI) | 40.5 (18, not estimable) 2.8, 41.5+ 93% (75, 98) 71% (51, 85) 67% (47, 82) 52% (26, 73) |
| Progression-free survival (PFS) | |
| Median PFS, months (95% CI) 6-month PFS rate by K-M, (95% CI) 12-month PFS rate by K-M, (95% CI) 24-month PFS rate by K-M, (95% CI) 36-month PFS rate by K-M, (95% CI) | 2.7 (1.4, 6.9) 40% (29, 50) 29% (19, 39) 26% (17, 36) 21% (12, 32) |
CI: Confidence interval; RECIST: Response Evaluation Criteria in Solid Tumours; IERC: Independent Endpoint Review Committee; K-M: Kaplan-Meier; +denotes a censored value
* Efficacy data with a minimum follow-up of 36 months (cut-off date 14 September 2018)
** CR or PR was confirmed at a subsequent tumour assessment
a Based on number of patients with confirmed response (CR or PR)
The median time to response was 6 weeks (range: 6 weeks to 36 weeks) after the first dose of avelumab. Twenty-two out of 29 (76%) patients with response were reported to have responded within 7 weeks after the first dose of avelumab.
The Kaplan-Meier estimates of PFS of the 88 patients (Part A) with metastatic MCC is presented in Figure 1.
Figure 1. Kaplan-Meier estimates of progression-free survival (PFS) per RECIST v1.1, IERC (Part A, minimum follow-up of 36 months):
Tumour samples were evaluated for PD-L1 tumour cell expression, and for Merkel cell polyomavirus (MCV) using an investigational immunohistochemistry (IHC) assay. Table 5 summarises the objective response rates by the PD-L1 expression and MCV status of patients with metastatic MCC in study EMR100070-003 (Part A).
Table 5. Objective response rates by PD-L1 expression and MCV tumour status in patients with metastatic MCC in study EMR100070-003 (Part A):
| Avelumab ORR (95% CI)* | |
|---|---|
| PD-L1 expression at cut-off of ≥1% | N=74a |
| Positive (n=58) | 36.2% (24.0, 49.9) |
| Negative (n=16) | 18.8% (4.0, 45.6) |
| IHC-MCV tumour status | N=77b |
| Positive (n=46) | 28.3% (16.0, 43.5) |
| Negative (n=31) | 35.5% (19.2, 54.6) |
IHC: Immunohistochemistry; MCV: Merkel cell polyomavirus; ORR: objective response rate
* ORR (cut-off date 14 September 2018)
a Based on data from patients evaluable for PD-L1
b Based on data from patients evaluable for MCV by immunohistochemistry (IHC)
Study 003 Part B – patients who have not received systemic therapy in the metastatic setting
The major efficacy outcome measure was durable response, defined as objective response (complete response (CR) or partial response (PR)) with a duration of at least 6 months; secondary outcome measures included BOR, DOR, PFS, and OS.
The primary analysis for Part B included 116 patients who received at least one dose of avelumab with a minimum follow-up of 15 months at the time of the data cut-off (cut-off date 02 May 2019).
Of the 116 patients, 81 (70%) were male, the median age was 74 years (range: 41 to 93 years), 75 (65%) were white, and 72 (62%) and 44 (38%) had an ECOG performance status of 0 and 1 respectively.
Table 6 summarises the primary analysis of efficacy endpoints including an estimate of the 24-month rates by Kaplan-Meier for DOR, and PFS in patients receiving avelumab at the recommended dose for study EMR100070-003, Part B.
Table 6. Primary analysis of response to avelumab 10 mg/kg every 2 weeks in patients with metastatic MCC in study EMR100070-003 (Part B)*:
| Efficacy endpoints (Part B) (per RECIST v1.1, IERC) | Results (N=116) |
|---|---|
| Durable response | |
| ≥6 months (95% CI) | 30.2% (22.0, 39.4) |
| Objective response rate (ORR) | |
| Response rate, CR+PR** n () (95 CI) | 46 (39.7%) (30.7, 49.2) |
| Confirmed best overall response (BOR) | |
| Complete response (CR)** n () Partial response (PR)** n () | 19 (16.4%) 27 (23.3%) |
| Duration of response (DOR)a | |
| Median, months (95% CI) Minimum, maximum (months) ≥3 months by K-M, (95% CI) ≥6 months by K-M, (95% CI) ≥12 months by K-M, (95% CI) ≥18 months by K-M, (95% CI) ≥24 months by K-M, (95% CI) | 18.2 (11.3, not estimable) 1.2, 28.3 89% (75, 95) 78% (63, 87) 66% (50, 78) 52% (34, 67) 45% (25, 63) |
| Progression-free survival (PFS) | |
| Median PFS, months (95% CI) 3-month PFS rate by K-M, (95% CI) 6-month PFS rate by K-M, (95% CI) 12-month PFS rate by K-M, (95% CI) 24-month PFS rate by K-M, (95% CI) | 4.1 (1.4, 6.1) 51% (42, 60) 41% (32, 50) 31% (23, 40) 20% (12, 30) |
CI: Confidence interval; RECIST: Response Evaluation Criteria in Solid Tumours; IERC: Independent Endpoint Review Committee; K-M: Kaplan-Meier
* Efficacy data with a minimum follow-up of 15 months (cut-off date 02 May 2019)
** CR or PR was confirmed at a subsequent tumour assessment
a Based on number of patients with confirmed response (CR or PR)
Figure 2 presents the Kaplan-Meier estimates for PFS from the primary analysis with 116 patients enrolled into Part B with a minimum follow-up of 15 months.
Figure 2. Kaplan-Meier estimates of progression-free survival (PFS) per RECIST v1.1, IERC (Part B, N=116):
Tumour samples were evaluated for PD-L1 tumour cell expression, and for MCV using an investigational IHC assay. Table 7 summarises the objective response rates by PD-L1 expression and MCV status of patients with metastatic MCC in study EMR100070-003 (Part B).
Table 7. Objective response rates by PD-L1 expression and MCV tumour status in patients with metastatic MCC in study EMR100070-003 (Part B):
| Avelumab ORR (95% CI)* | |
|---|---|
| PD-L1 expression at cut-off of ≥1% | N=108a |
| Positive (n=21) | 61.9% (38.4, 81.9) |
| Negative (n=87) | 33.3% (23.6, 44.3) |
| IHC-MCV tumour status | N=107b |
| Positive (n=70) | 34.3% (23.3, 46.6) |
| Negative (n=37) | 48.6% (31.9, 65.6) |
IHC: Immunohistochemistry; MCV: Merkel cell polyomavirus; ORR: objective response rate
* ORR (cut-off date 02 May 2019)
a Based on data from patients evaluable for PD-L1
b Based on data from patients evaluable for MCV by IHC
Locally advanced or metastatic urothelial carcinoma (study B9991001)
The efficacy and safety of avelumab was demonstrated in study B9991001, a randomised, multi-centre, open-label study conducted in 700 patients with unresectable, locally advanced or metastatic urothelial carcinoma whose disease had not progressed with 4-6 cycles of first-line platinum-based induction chemotherapy. Patients with autoimmune disease or a medical condition that required immunosuppression were excluded.
Randomization was stratified by best response to chemotherapy (CR/PR vs. stable disease [SD]) and site of metastasis (visceral vs. non-visceral) at the time of initiating first-line induction chemotherapy. Patients were randomised (1:1) to receive either avelumab 10 mg/kg intravenous infusion every 2 weeks plus best supportive care (BSC) or BSC alone.
Administration of avelumab was permitted beyond Response Evaluation Criteria in Solid Tumours (RECIST) v1.1-defined progression of disease by Blinded Independent Central Review (BICR) if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumour status was performed at baseline, 8 weeks after randomization, then every 8 weeks up to 12 months after randomization, and every 12 weeks thereafter until documented confirmed disease progression based on BICR assessment per RECIST v1.1.
Demographic and baseline characteristics were generally well balanced between the avelumab plus BSC and the BSC alone arm. Baseline characteristics were a median age of 69 years (range: 32 to 90), 66% of patients were 65 years or older, 77% were male, 67% were White, and the ECOG PS was 0 (61%) or 1 (39%) for both arms.
For first-line induction chemotherapy, 56% of patients received cisplatin plus gemcitabine, 38% of patients received carboplatin plus gemcitabine and 6% of patients received cisplatin plus gemcitabine and carboplatin plus gemcitabine (i.e. these patients received one or more cycles of each combination). Best response to first-line induction chemotherapy was CR or PR (72%) or SD (28%). Sites of metastasis prior to chemotherapy were visceral (55%) or non-visceral (45%). Fifty-one percent of patients had PD-L1-positive tumours. Six percent of patients in the avelumab plus BSC arm and 44% of patients in the BSC alone arm received another PD-1/PD-L1 checkpoint inhibitor after discontinuation of treatment.
The primary efficacy outcome measure was overall survival (OS) in all randomized patients and in patients with PD-L1-positive tumours. Progression-free survival (PFS) based on BICR assessment per RECIST v1.1 was an additional efficacy outcome measure. Efficacy outcomes were measured from time of randomisation after 4 to 6 cycles of platinum-based induction chemotherapy.
The PD-L1 status of the tumour was assessed using the Ventana PD-L1 (SP263) assay. PD-L1-positivity was defined as ≥25% of tumour cells stained for PD-L1; or ≥25% of immune cells stained for PD-L1 if >1% of the tumour area contained immune cells; or 100% of immune cells stained for PD-L1 if = 1% of the tumour area contained immune cells.
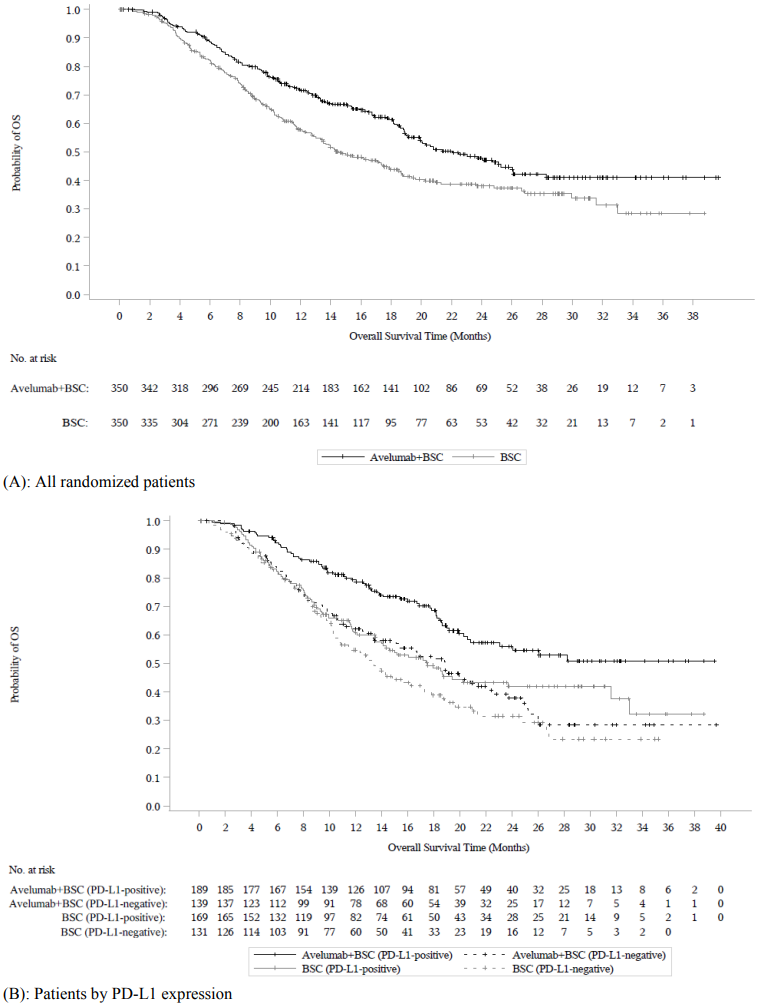
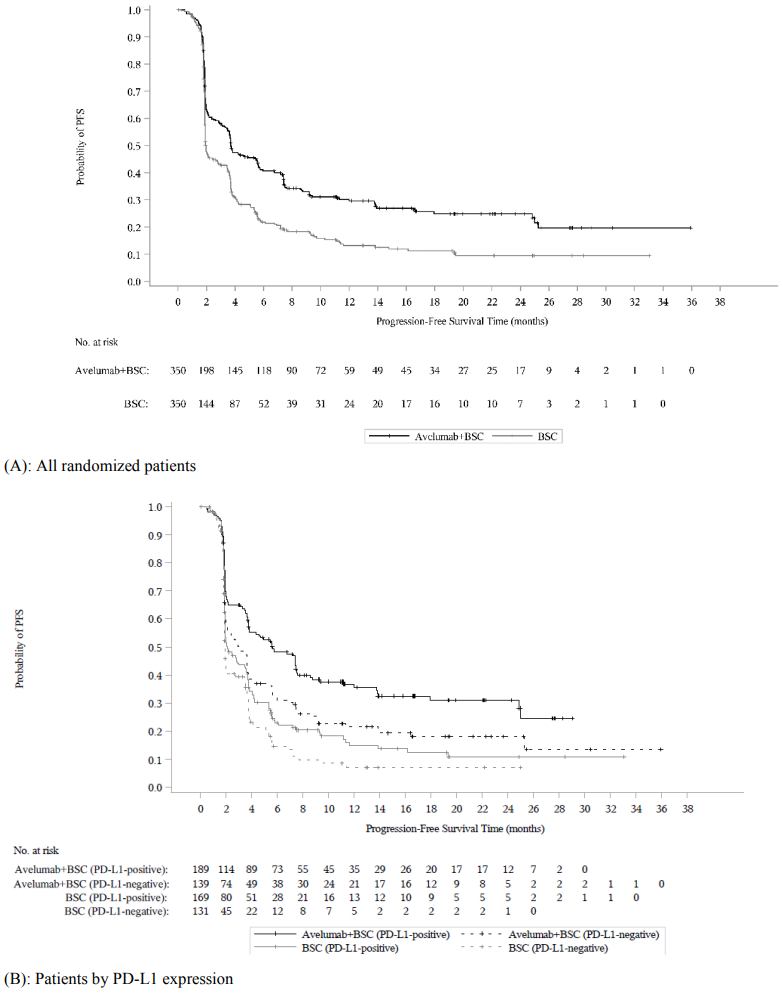
At the pre-specified interim analysis (cut-off date 21 October 2019), study B9991001 met its primary endpoint for OS in both coprimary populations: in all randomized patients with a median OS of 21.4 months (95% CI: 18.9, 26.1; HR 0.69, 95% CI: 0.556, 0.863) in the avelumab plus BSC arm and with a median OS of 14.3 months (95% CI: 12.9, 17.8) in the BSC alone arm. For patients with PD-L1-positive tumours the median OS was not reached (95% CI: 20.3, not reached; HR 0.56, 95%, CI: 0.404, 0.787) in the avelumab plus BSC arm and the median OS in the BSC alone arm was 17.1 months (95% CI: 13.5, 23.7). Updated OS results with a data cut-off date of 19 January 2020 and PFS data with a cut-off date of 21 October 2019 are presented in Table 8 and in Figure 3 and Figure 4 below.
Table 8. Efficacy results by PD-L1 expression in study B9991001:
| Efficacy endpoints | Avelumab plus BSC | BSC | Avelumab plus BSC | BSC | velumab plus BSC | BSC |
|---|---|---|---|---|---|---|
| (N=350) | (N=350) | (N=189) | (N=169) | (N=139) | (N=131) | |
| All randomized patients | PD-L1-positive tumours | PD-L1-negative tumoursc | ||||
| Overall survival (OS)a | ||||||
| Events (%) | 156 (44.6) | 190 (54.3) | 68 (36.0) | 85 (50.3) | 80 (57.6) | 80 (61.1) |
| Median in months | 22.1 | 14.6 | NE | 17.5 | 18.9 | 13.4 |
| (95% CI) | (19.0, 26.1) | (12.8, 17.8) | (20.6, NE) | (13.5, 31.6) | (13.3, 22.1) | (10.4, 17.3) |
| Hazard ratio | 0.70 | 0.60 | 0.83 | |||
| (95% CI) | (0.564, 0.862) | (0.439, 0.833) | (0.603, 1.131) | |||
| 2-sided p-valued | 0.0008 | 0.0019 | - | |||
| Progression-free survival (PFS)b,e,f | ||||||
| Events (%) | 225 (64.3) | 260 (74.3) | 109 (57.7) | 130 (76.9) | 103 (74.1) | 99 (75.6) |
| Median in months | 3.7 | 2.0 | 5.7 | 2.1 | 3.0 | 1.9 |
| (95% CI) | (3.5, 5.5) | (1.9, 2.7) | (3.7, 7.4) | (1.9, 3.5) | (2.0, 3.7) | (1.9, 2.1) |
| Hazard ratio | 0.62 | 0.56 | 0.63 | |||
| (95% CI) | (0.519, 0.751) | (0.431, 0.728) | (0.474, 0.847) | |||
| 2-sided p-valued | <0.0001 | <0.0001 | - | |||
CI: Confidence interval; K-M: Kaplan-Meier, NE: not estimable
Note: 72 patients (22 patients on avelumab plus BSC arm and 50 patients on BSC alone arm) had a tumour with an unknown PD-L1 status
a OS cut-off date 19 January 2020
b PFS cut-off date 21 October 2019
c PD-L1-negative population analyses were exploratory and no formal test was performed
d p-value based on stratified log-rank
e Based on BICR assessment per RECIST v1.1
f PFS censoring reasons follow the hierarchy in sequential order: no adequate baseline assessment, start of new anti-cancer therapy, event after 2 or more missing assessments, withdrawal of consent, lost to follow-up, no adequate post-baseline tumour assessment, ongoing without an event
Figure 3. Kaplan-Meier estimates for overall survival (OS) by PD-L1 expression (cut-off date 19 January 2020) - Full analysis set:
Figure 4. Kaplan-Meier estimates for progression-free survival (PFS) by PD-L1 expression based on BICR assessment (RECIST v1.1) (cut-off date 21 October 2019) - Full analysis set:
Renal cell carcinoma (study B9991003)
The efficacy and safety of avelumab in combination with axitinib was demonstrated in study B9991003, a randomised, multi-centre, open-label study of avelumab in combination with axitinib in 886 patients with untreated advanced or metastatic RCC with a clear-cell component.
Patients were included irrespective of prognostic risk groups or tumour PD-L1 expression and had to have at least one measurable lesion as defined by Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 that was not been previously irradiated. Patients with prior systemic therapy directed at advanced or metastatic RCC; prior systemic immunotherapy treatment with IL-2, IFN-α, anti-PD-1, anti-PD-L1, or anti-CTLA-4 antibodies, or active brain metastasis; active autoimmune disease that might deteriorate when receiving an immunostimulatory agents; a history of other malignancies within the last 5 years; organ transplant were ineligible.
Randomization was stratified according to Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) (0 vs. 1) and region (United States vs. Canada/Western Europe vs. the rest of the world). Patients were randomised (1:1) to one of the following treatment arms:
- Avelumab 10 mg/kg intravenous infusion every 2 weeks in combination with axitinib 5 mg twice daily orally (N=442). Patients who tolerated axitinib 5 mg twice daily without Grade 2 or greater axitinib-related adverse events for 2 consecutive weeks could increase to 7 mg and then subsequently to 10 mg twice daily. Axitinib could be interrupted or reduced to 3 mg twice daily and subsequently to 2 mg twice daily to manage toxicity.
- Sunitinib 50 mg once daily orally for 4 weeks followed by 2 weeks off (N=444) until radiographic or clinical progression or unacceptable toxicity.
Treatment with avelumab and axitinib continued until RECIST v1.1-defined progression of disease by Blinded Independent Central Review (BICR) assessment or unacceptable toxicity. Administration of avelumab and axitinib was permitted beyond RECIST-defined disease progression based on investigator’s assessment of the patient’s benefit-risk and clinical condition, including performance status, clinical symptoms, adverse events and laboratory data. The majority (n=160, 71.4%) of the patients with progressive disease continued treatment with both medicinal products after progression. Assessment of tumour status was performed at baseline, after randomisation at 6 weeks, then every 6 weeks thereafter up to 18 months after randomisation, and every 12 weeks thereafter until documented confirmed disease progression by BICR.
The primary efficacy endpoints were progression-free survival (PFS), as assessed by BICR using RECIST v1.1 and overall survival (OS) in the first-line treatment of patients with advanced RCC who have PD-L1-positive tumours (PD-L1 expression level ≥1%). The key secondary endpoints were PFS based on BICR assessment per RECIST v1.1 and OS irrespective of PD-L1 expression. PD-L1 status was determined by immunohistochemistry. Additional secondary endpoints included objective response (OR), time to response (TTR) and duration of response (DOR).
Study population characteristics: median age of 61 years (range: 27.0 to 88.0), 38% of patients were 65 years or older, 75% were male, 75% were White, and the ECOG performance score was 0 (63%) or 1 (37%).
Patient distribution by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk groups was 21% favourable, 62% intermediate, and 16% poor. Patient distribution by Memorial Sloan–Kettering Cancer Center (MSKCC) risk groups was 22% favourable, 65% intermediate, and 11% poor.
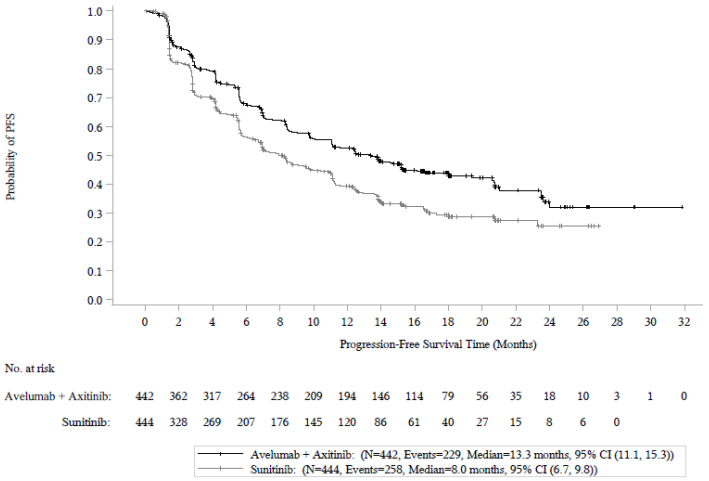
Efficacy results are presented in Table 9 and Figure 5 based on a data cut-off date of 28 January 2019. With a median OS follow-up of 19 months, OS data were immature with 27% deaths. The observed hazard ratio (HR) for OS was 0.80 (95% CI: 0.616, 1.027) for avelumab in combination with axitinib compared to sunitinib.
Table 9. Efficacy results from study B9991003 in patients irrespective of PD-L1 expression:
| Efficacy endpoints (Based on BICR assessment) | Avelumab plus axitinib (N=442) | Sunitinib (N=444) |
|---|---|---|
| Progression-free survival (PFS) | ||
| Events (%) | 229 (52) | 258 (58) |
| Median in months (95% CI) | 13.3 (11.1, 15.3) | 8.0 (6.7, 9.8) |
| Hazard ratio (95% CI) | 0.69 (0.574, 0.825) | |
| p-value* | <0.0001 | |
| 12-month PFS rate by K-M, (95% CI)** | 52.4% (47.4, 57.2) | 39.2% (34.1, 44.2) |
| 18-month PFS rate by K-M, (95% CI)** | 43.9% (38.8, 49.0) | 29.3% (24.2, 34.6) |
| Confirmed objective response rate (ORR) | ||
| Objective response rate (ORR) n (%) | 232 (52.5) | 121 (27.3) |
| (95% CI) | 47.7, 57.2 | 23.2, 31.6 |
| Complete response (CR) n (%) | 17 (3.8) | 9 (2.0) |
| Partial response (PR) n (%) | 215 (48.6) | 112 (25.2) |
| Time to response (TTR) | ||
| Median, months (range) | 2.7 (1.2, 20.7) | 4.0 (1.2, 18.0) |
| Duration of response (DOR) | ||
| Median, months (95% CI) | 18.5 (17.8, NE) | NE (16.4, NE) |
BICR: Blinded Independent Central Review; CI: Confidence interval; K-M: Kaplan-Meier; NE: Not estimable
* 1-sided p-value based on stratified log-rank
** CIs are derived using the log-log transformation with back transformation to untransformed scale
Figure 5. Kaplan-Meier estimates for progression-free survival based on BICR assessment in patients irrespective of PD-L1 expression:
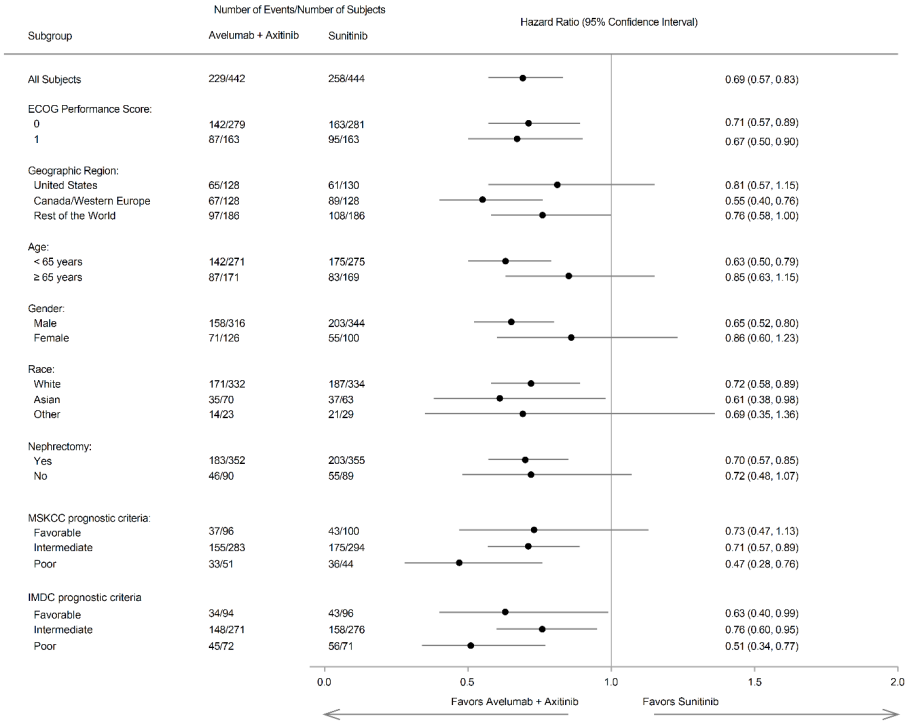
Improvement of PFS was observed across pre-specified subgroups.
Figure 6. Forest plot of progression-free survival based on BICR assessment in patients irrespective of PD-L1 expression:
Paediatric population
Study MS100070-0306 was a multi-centre, open-label, Phase I/II study to evaluate the dose, safety and tolerability, antitumour activity, pharmacokinetic, and pharmacodynamics of avelumab in paediatric patients from birth to less than 18 years of age with refractory or relapsed solid tumours including central nervous system (CNS) tumours and lymphoma for which no standard therapy is available or for which the patient was not eligible for the existing therapy.
The study enrolled 21 paediatric patients with an age ranged from 3 to 17 years (11 patients ≤12 years and 10 patients >12 years) receiving either 10 mg/kg (N=6) or 20 mg/kg (N=15) avelumab intravenously every 2 weeks until confirmed progression, death, or unacceptable toxicity.
The primary tumour categories were soft tissue/bone sarcoma (N=12), CNS malignancies (N=8), and gastro-intestinal (GI) carcinoma (N=1).
There was no complete response (CR) or partial response (PR) in this study as assessed according to RECIST 1.1.
The European Medicines Agency has waived the obligation to submit the results of studies with Bavencio in all subsets of the paediatric population for the treatment of Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Avelumab pharmacokinetics (PK) was assessed using a population PK approach for avelumab as monotherapy and avelumab in combination with axitinib.
Based on a population PK analysis for avelumab as monotherapy and in combination with axitinib, there are no expected clinically meaningful differences in exposure of avelumab between settings administered every 2 weeks at 800 mg or 10 mg/kg.
Distribution
Avelumab is expected to be distributed in the systemic circulation and to a lesser extent in the extracellular space. The volume of distribution at steady state was 4.72 L.
Consistent with a limited extravascular distribution, the volume of distribution of avelumab at steady state is small. As expected for an antibody, avelumab does not bind to plasma proteins in a specific manner.
Elimination
Based on a population pharmacokinetic analysis from 1 629 patients, the value of total systemic clearance (CL) is 0.59 L/day. In the supplemental analysis, avelumab CL was found to decrease over time: the largest mean maximal reduction (% coefficient of variation [CV%]) from baseline value with different tumour types was approximately 32.1% (CV 36.2%).
Steady-state concentrations of avelumab were reached after approximately 4 to 6 weeks (2 to 3 cycles) of repeated dosing at 10 mg/kg every 2 weeks, and systemic accumulation was approximately 1.25-fold.
The elimination half-life (t½) at the recommended dose is 6.1 days based on the population PK analysis.
Linearity/non-linearity
The exposure of avelumab increased dose-proportionally in the dose range of 10 mg/kg to 20 mg/kg every 2 weeks.
When avelumab 10 mg/kg was administered in combination with axitinib 5 mg, the respective exposures of avelumab and axitinib were unchanged compared to the single agents. There was no evidence to suggest a clinically relevant change of avelumab clearance over time in patients with advanced RCC.
Special populations
A population pharmacokinetic analysis suggested no difference in the total systemic clearance of avelumab based on age, gender, race, PD-L1 status, tumour burden, renal impairment and mild or moderate hepatic impairment.
Total systemic clearance increases with body weight. Steady-state exposure was approximately uniform over a wide range of body weights (30 to 204 kg) for body weight normalised dosing.
Renal impairment
No clinically important differences in the clearance of avelumab were found between patients with mild (glomerular filtration rate (GFR) 60 to 89 mL/min, Cockcroft-Gault Creatinine Clearance (CrCL); n=623), moderate (GFR 30 to 59 mL/min, n=320) and patients with normal (GFR ≥90 mL/min, n=671) renal function.
Avelumab has not been studied in patients with severe renal impairment (GFR 15 to 29 mL/min).
Hepatic impairment
No clinically important differences in the clearance of avelumab were found between patients with mild hepatic impairment (bilirubin ≤ ULN and AST > ULN or bilirubin between 1 and 1.5 times ULN, n=217) and normal hepatic function (bilirubin and AST ≤ ULN, n=1 388) in a population PK analysis. Hepatic impairment was defined by National Cancer Institute (NCI) criteria of hepatic dysfunction.
Avelumab has not been studied in patients with moderate hepatic impairment (bilirubin between 1.5 and 3 times ULN) or severe hepatic impairment (bilirubin >3 times ULN).
Paediatric population
The pharmacokinetics of avelumab was evaluated in 21 children and adolescents from 3 years to 17 years in study MS100070-0306 receiving either 10 mg/kg (N=6) or 20 mg/kg (N=15) avelumab intravenously every 2 weeks until confirmed progression, death, or unacceptable toxicity.
The paediatric PK parameters and the corresponding PK profiles for all patients were evaluated according to dosing and stratified by bodyweight.
The exposure in paediatric patients receiving 20 mg/kg avelumab were similar or higher compared to those in adults receiving 10 mg/kg or 800 mg avelumab. In paediatric patients receiving 10 mg/kg avelumab the exposure was lower compared to those in adults.
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of repeated dose toxicity in Cynomolgus monkeys administered intravenously doses of 20, 60 or 140 mg/kg once a week for1 month and 3 months, followed by a 2-month recovery period after the 3-month dosing period. Perivascular mononuclear cell cuffing was observed in the brain and spinal cord of monkeys treated with avelumab at ≥20 mg/kg for 3 months. Although there was no clear dose-response relationship, it cannot be excluded that this finding was related to avelumab treatment.
Animal reproduction studies have not been conducted with avelumab. The PD-1/PD-L1 pathway is thought to be involved in maintaining tolerance to the foetus throughout pregnancy. Blockade of PD-L1 signalling has been shown in murine models of pregnancy to disrupt tolerance to the foetus and to result in an increase in foetal loss. These results indicate a potential risk that administration of avelumab during pregnancy could cause foetal harm, including increased rates of abortion or stillbirth.
No studies have been conducted to assess the potential of avelumab for carcinogenicity or genotoxicity.
Fertility studies have not been conducted with avelumab. In 1-month and 3-month repeat-dose toxicology studies in monkeys, there were no notable effects in the female reproductive organs. Many of the male monkeys used in these studies were sexually immature and thus no explicit conclusions regarding effects on male reproductive organs can be made.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.