BELKYRA Solution for injection Ref.[7699] Active ingredients: Deoxycholic acid
Source: Health Products Regulatory Authority (IE) Revision Year: 2019 Publisher: Allergan Pharmaceuticals International Limited, Clonshaugh Business & Technology park, Dublin 17, D17 E400, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Other dermatological preparations
ATC code: D11AX24
Mechanism of action
Deoxycholic acid is a cytolytic drug, which when injected into localized subcutaneous fat, physically disrupts the cell membrane of adipocytes. The destruction of adipocytes elicits a tissue response in which macrophages are attracted to the area to eliminate cellular debris and lipids, which are then cleared through natural processes. This is followed by the appearance of fibroblasts and observed thickening of fibrous septa suggesting an increase in total collagen (i.e. neocollagenesis).
Clinical efficacy and safety
Four Phase 3 randomized, multi-center, double-blind, placebo-controlled trials were conducted (2 identical studies conducted in the European Union [EU] and 2 identical trials conducted in North America) to evaluate Belkyra for the treatment of convexity or fullness associated with submental fat (SMF) and the assessment of the associated psychological impact. In all trials the primary endpoints were measured 12 weeks after final treatment. Each Phase 3 trial met its primary efficacy endpoints, and showed improvement in psychological impact versus placebo.
The trials enrolled adults (ages 19 to 65) with moderate or severe convexity or fullness associated with SMF (i.e. grade 2 or 3 on 5-point grading scales, where 0 = absent, 4 = extreme), as judged by both clinician and subject ratings. Patients received up to 4 treatments in the trials conducted in the EU, and up to 6 treatments in the trials conducted in North America, with Belkyra (N=757 for all 4 studies) or placebo (N=746) at 28-day intervals. Treatment was stopped when the desired response was achieved. Injection volume was 0.2 ml per injection site, spaced 1 cm apart into the SMF tissue, which is also expressed in dose per area as 2 mg/cm². For each treatment session a maximum of 100 mg (10 ml) was permitted over the entire treatment area.
The mean age in the trials conducted in the EU was 46 years and the mean BMI was 26. Most patients were women (75%) and Caucasian (94%). At baseline, 68% of the patients had a clinician-rated SMF severity rating of moderate and 32% had a severe SMF rating. For trials conducted in North America, the mean age was 49 years and the mean BMI was 29 kg/m². Most of the patients were women (85%) and Caucasian (87%). At baseline, 51% of the patients had a clinician-rated SMF severity rating of moderate and 49%% had a severe SMF rating.
The co-primary efficacy assessments in the EU trials were the clinician-reported ratings of SMF (CR-SMFRS) and patient assessment of satisfaction (Subject Self Rating Scale [SSRS]). Patient–reported rating of SMF (PR-SMFRS) was also assessed. Psychological impact of SMF was evaluated using multiple measures including the Derriford Appearance Scale-24 (DAS-24), the Body Image Quality of Life Inventory (BIQLI) and the Patient Reported–Submental Fat Impact Scale (PR-SMFIS) a 6-item questionnaire (assessing happiness, bothersomeness, self-consciousness, embarrassment, looking older or overweight). Statistically significant improvements in clinician- and patient-rated SMF, patient satisfaction and reduction in psychological impact of SMF were observed more frequently in the Belkyra group compared to the placebo group (Table 1). Reduction in submental fat volume was confirmed by caliper measurements.
In the studies conducted in North America, the co-primary efficacy assessments were based on at least 2-grade and at least 1-grade improvements in submental convexity or fullness on the composite of clinician-reported (CR-SMFRS) and patient-reported (PR-SMFRS) ratings of submental fat 12 weeks after final treatment. Psychological impact of SMF was assessed using the same 6-item questionnaire as in the EU trials. In addition, changes in submental fat volume were evaluated in a subset of patients (N=449, combined trials) using magnetic resonance imaging (MRI). Reduction in submental fat volume was confirmed by both MRI and caliper measurements.
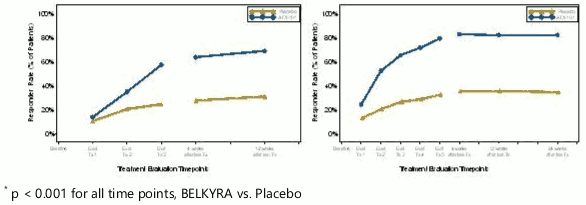
Table 1. below displays 1-Grade Clinician Response (CR-SMFRS), Patient Satisfaction Response (SSRS), and Psychological Impact (PR-SMFIS) improvement as applied to all four Phase 3 trials. Figure 4 provides the response rates based on clinician SMF ratings at each study visit:
| Trials conducted in the EUa | Trials conducted in North Americab | |||
|---|---|---|---|---|
| Endpoint | BELKYRA (N=243) | Placebo (N=238) | BELKYRA (N=514) | Placebo (N=508) |
| 1-Grade Clinician Response (CR-SMFRS)c | 63.8% | 28.6% | 78.5% | 35.3% |
| 1-Grade Patient Response (PR-SMFRS)c | 63.1% | 34.3% | 80.3% | 38.1% |
| Patient Satisfaction Response (SSRS)d | 65.4% | 29% | 69.1% | 30.5% |
| Psychological Impact (PR-SMFIS) Percent Mean Improvement from Baselinee | 44.6% | 18.0% | 48.6% | 17.3% |
a Up to 4 treatment sessions permitted
b Up to 6 treatment sessions permitted
c At least a 1-grade reduction in the clinician-reported ratings (CR-SMFRS) of SMF 12 weeks after last treatment
d A patient rating of “extremely satisfied”, “satisfied” or “slightly satisfied” on the SSRS 12 weeks after last treatment
e Percent mean improvement from baseline calculated as the PR-SMFIS mean change from baseline divided by the baseline mean
Figure 4. Clinician SMF Rating (CR-SMFRS) 1-Grade Responder Rates at Each Study Visit; Pooled Data From EU Trials (Left Panel) and North America Trials (Right Panel):
Despite the majority of patients having reductions in SMF volumes, 90.0% and 92% of patients in the EU and US/Canada trials respectively, had no change (68.9% and 70.5%) or an improvement (21.6% and 22.9%) in skin laxity scores 12 weeks after last treatment compared with baseline.
The long-term safety and maintenance of treatment effect has been assessed following treatment with Belkyra. A subset of the initial Belkyra-treated responders continued in these follow-up studies, where maintenance of treatment effect has been demonstrated for up to 5 years.
Paediatric population
The use of Belkyra is not recommended in individuals under 18 years.
The European Medicines Agency has waived the obligation to submit the results of studies with Belkyra in all subsets of the paediatric population in treatment of moderate to severe convexity or fullness associated with submental fat in adults when the presence of submental fat has a psychological impact for the patient (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Endogenous deoxycholic acid plasma levels are highly variable within and between individuals; most of this natural secondary bile acid is sequestered in the enterohepatic circulation system. Pharmacokinetics of exogenous deoxycholic acid administered via treatment with Belkyra was compared against this endogenous background.
Absorption
Deoxycholic acid from Belkyra is rapidly absorbed following subcutaneous injection. After dosing with the maximum recommended single treatment with Belkyra (100 mg), maximum plasma concentrations (mean Cmax) were observed with a median tmax of 6 minutes after injection. The mean Cmax value was 1036 ng/ml and was 2.3-fold higher than average Cmax values observed during a 24-hour baseline endogenous period in the absence of Belkyra. At the maximum recommended single treatment dose (100 mg), deoxycholic acid exposure (AUC0-24) was less than 2-fold higher over endogenous exposure.
Plasma AUC0-24 increased in a dose-proportional manner up to 100 mg. Post-treatment deoxycholic acid plasma levels returned to the endogenous range within 24 hours. No accumulation is expected with the proposed treatment frequency.
Distribution
The volume of distribution was estimated to be 193 L and is independent of the dose up to 100 mg. Deoxycholic acid is extensively bound to proteins in plasma (98%).
Elimination
Endogenous deoxycholic acid is a product of cholesterol metabolism and is excreted intact in feces. Deoxycholic acid from Belkyra joins the endogenous bile acid pool and is excreted along with the endogenous deoxycholic acid. Deoxycholic acid is eliminated via hepatic transport proteins from the blood to the bile without any significant contribution of metabolism. Deoxycholic acid is not an in vitro inhibitor of the enzymes CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4. Deoxycholic acid did not induce CYP1A, 2B6 and 3A at a clinically level.
Deoxycholic acid is not an in vitro inhibitor of the transporters BSEP, MRP2, MRP4, MDR1, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, OATP2B1 and ASBT. Deoxycholic acid inhibited NTCP with an IC50 of 2.14 µM in vitro.
Renal impairment
Belkyra has not been studied in patients with renal impairment. Bile acids including deoxycholic acid are excreted in the urine in negligible amounts; renal impairment is unlikely to influence deoxycholic acid pharmacokinetics.
Hepatic impairment
Belkyra has not been studied in patients with hepatic impairment. Considering the intermittent dose frequency, the small dose administered that represents approximately 3% of the total bile acid pool, and the highly variable endogenous deoxycholic acid levels, the pharmacokinetics of deoxycholic acid following Belkyra injection is unlikely to be influenced by hepatic impairment.
Elderly
No dose adjustment is considered necessary. Caution should be exercised in elderly patients (see section 4.4).
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction and development.
Carcinogenicity
In repeat dose toxicity studies of up to 6 months in rats and 9 months duration in dogs, there was no indication of local or systemic pre-neoplastic responses to subcutaneous Belkyra administration. In these studies, the maximum intended clinical dose was exceeded by 2.5 to 12.5-fold (based on mg/injection site) and 2 to 3-fold (based on quantified systemic exposure) in rats and dogs, respectively. Further, in contrast to the maximum intended clinical regimen of monthly injections for up to 6 sessions, Belkyra injections were administered twice monthly for up to 13 total doses in rats and 20 total doses in dogs. No carcinogenicity studies have been conducted with Belkyra.
Genotoxicity
Belkyra was negative in a standard battery of in vitro (microbial reverse mutation assay and chromosomal aberration assay) and in vivo (micronucleus assay) genetic toxicology assays.
Developmental toxicity
Inconclusive findings of missing intermediate lung lobe were noted in rabbits in the embryo-fetal toxicity study. The finding was significantly increased in the 30mg/kg group but was evident also at the lowest concentration 10mg/kg. This dose was associated with maternal local toxicity. The clinical significance of the finding is unclear.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.