BENLYSTA Solution for injection Ref.[6410] Active ingredients: Belimumab
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: GlaxoSmithKline (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, monoclonal antibodies
ATC code: L04AG04
Mechanism of action
Belimumab is a human IgG1λ monoclonal antibody specific for soluble human B Lymphocyte Stimulator protein (BLyS, also referred to as BAFF and TNFSF13B). Belimumab blocks the binding of soluble BLyS, a B cell survival factor, to its receptors on B cells. Belimumab does not bind B cells directly, but by binding BLyS, belimumab inhibits the survival of B cells, including autoreactive B cells, and reduces the differentiation of B cells into immunoglobulin-producing plasma cells.
BLyS levels are elevated in patients with SLE and other autoimmune diseases. There is an association between plasma BLyS levels and SLE disease activity. The relative contribution of BLyS levels to the pathophysiology of SLE is not fully understood.
Pharmacodynamic effects
Median IgG levels at Week 52 were reduced by 11% in patients with SLE receiving Benlysta compared with an increase of 0.7% in patients receiving placebo.
In patients with anti-dsDNA antibodies at baseline, median anti-dsDNA antibodies levels at Week 52 were reduced by 56% in patients receiving Benlysta compared with 41% in patients receiving placebo. In patients with anti-dsDNA antibodies at baseline, by Week 52, 18% of patients treated with Benlysta had converted to anti-dsDNA negative compared with 15% of the patients receiving placebo.
In patients with SLE with low complement levels, normalization of C3 and C4 was observed by Week 52 in 42% and 53% of patients receiving Benlysta and in 21% and 20% of patients receiving placebo, respectively.
Benlysta significantly reduced circulating overall, transitional, naïve, and SLE B cells, as well as plasma cells at Week 52. Reductions in naïve and transitional B cells, as well as the SLE B cell subset were observed as early as Week 8. Memory cells increased initially and slowly declined toward baseline levels by Week 52.
The B cell and IgG response to long term treatment with intravenous Benlysta was assessed in an uncontrolled SLE extension study. After 7 and a half years of treatment (including the 72-week parent study), a substantial and sustained decrease in various B cell subsets was observed leading to 87% median reduction in naïve B cells, 67% in memory B cells, 99% in activated B cells, and 92% median reduction in plasma cells after more than 7 years of treatment. After about 7 years, a 28% median reduction in IgG levels was observed, with 1.6% of subjects experiencing a decrease in IgG levels to below 400 mg/dL. Over the course of the study, the reported incidence of AEs generally remained stable or declined.
In patients with active lupus nephritis, following treatment with Benlysta (10 mg/kg body weight intravenously) or placebo, there was an increase in serum IgG levels which was associated with decreased proteinuria. Relative to placebo, smaller increases in serum IgG levels were observed in the Benlysta group as expected with the known mechanism of belimumab. At Week 104, the median percent increase from baseline in IgG was 17% for Benlysta and 37% for placebo. Reductions in autoantibodies, increases in complement, and reductions in circulating total B cells and B-cell subsets observed were consistent with the SLE studies.
In one intravenous study in paediatric patients with SLE (6 to 17 years of age) and one subcutaneous study in paediatric patients with SLE (10 to 17 years of age), the pharmacodynamic response was consistent with the adult data.
Immunogenicity
In the subcutaneous study where serum samples from more than 550 adult patients with SLE were tested, no anti-belimumab antibodies were detected during or after treatment with belimumab 200 mg subcutaneously. In the lupus nephritis study where 224 adult patients received Benlysta 10 mg/kg body weight intravenously, no anti-belimumab antibodies were detected.
In one intravenous study in 6- to 17-year-old paediatric patients (n=53) with SLE and one subcutaneous study in 10- to 17-year-old paediatric patients (n=25) with SLE, none of the patients developed anti-belimumab antibodies.
Clinical efficacy and safety
SLE
Subcutaneous injection
The efficacy of Benlysta administered subcutaneously was evaluated in a randomised, double-blind, placebo-controlled 52-week Phase III study (HGS1006-C1115; BEL112341) in 836 adult patients with a clinical diagnosis of SLE according to the American College of Rheumatology classification criteria. Eligible patients had active SLE disease, defined as a SELENA-SLEDAI score ≥8 and positive anti-nuclear antibody (ANA or anti-dsDNA) test results (ANA titre ≥1:80 and/or a positive anti-dsDNA [≥30 units/mL]) at screening. Patients were on a stable SLE treatment regimen (standard of care) consisting of any of the following (alone or in combination): corticosteroids, anti-malarials, NSAIDs or other immunosuppressives. Patients were excluded from the study if they had severe active central nervous system lupus or severe active lupus nephritis.
This study was conducted in the US, South America, Europe and Asia. Patient median age was 37 years (range: 18 to 77 years), and the majority (94%) were female. Background medicinal products included corticosteroids (86%; >7.5 mg/day prednisone equivalent 60%), immunosuppressives (46%), and anti-malarials (69%). Patients were randomised in a 2:1 ratio to receive belimumab 200 mg or placebo subcutaneously once weekly for 52 weeks.
At baseline 62.2% of patients had high disease activity (SELENA SLEDAI score ≥10), 88% of patients had mucocutaneous, 78% had musculoskeletal, 8% had haematological, 12% had renal, and 8% had vascular organ involvement.
The primary efficacy endpoint was a composite endpoint (SLE Responder Index) that defined response as meeting each of the following criteria at Week 52 compared with baseline:
- ≥4-point reduction in the SELENA-SLEDAI score, and
- no new British Isles Lupus Assessment Group (BILAG) A organ domain score or 2 new BILAG B organ domain scores, and
- no worsening (<0.30 point increase) in Physician's Global Assessment score (PGA)
The SLE Responder Index measures improvement in SLE disease activity, without worsening in any organ system, or in the patient's overall condition.
Table 1. Response rate at Week 52:
| Response1 | Placebo2 (n=279) | Benlysta2 200 mg weekly (n=554) |
|---|---|---|
| SLE responder index Observed difference vs. placebo Odds ratio (95% CI) vs. placebo | 48.4% | 61.4% (p=0.0006) 12.98% 1.68 (1.25, 2.25) |
| Components of SLE responder index | ||
| Percent of patients with reduction in SELENA-SLEDAI ≥4 | 49.1% | 62.3% (p=0.0005) |
| Percent of patients with no worsening by BILAG index | 74.2% | 80.9% (p=0.0305) |
| Percent of patients with no worsening by PGA | 72.8% | 81.2% (p=0.0061) |
1 Analyses excluded any subject missing a baseline assessment for any of the components (1 for placebo; 2 for Benlysta).
2 All patients received standard therapy.
The differences between the treatment groups were apparent by Week 16 and sustained through Week 52 (Figure 1).
Figure 1. Proportion of SRI responders by visit:
Flares in SLE were defined by the modified SELENA SLEDAI SLE Flare Index. The risk of first flare was reduced by 22% during the 52 weeks of observation in the group receiving Benlysta compared with the group receiving placebo (hazard ratio = 0.78; p=0.0061). The median time to the first flare among patients having a flare was delayed in patients receiving Benlysta compared with placebo (190 days vs. 141 days). Severe flares were observed in 10.6% of patients in the group receiving Benlysta compared with 18.2% of patients in the group receiving placebo over the 52 weeks of observation (observed treatment difference = -7.6%). The risk of severe flares was reduced by 49% during the 52 weeks of observation in the group receiving Benlysta compared with the group receiving placebo (hazard ratio = 0.51; p=0.0004). The median time to the first severe flare among patients having a severe flare was delayed in patients receiving Benlysta compared with placebo (171 days vs. 118 days).
The percentage of patients receiving greater than 7.5 mg/day prednisone (or equivalent) at baseline whose average corticosteroid dose was reduced by at least 25% from baseline to a dose equivalent to prednisone ≤7.5 mg/day during Weeks 40 through 52, was 18.2% in the group receiving Benlysta and 11.9% in the group receiving placebo (p=0.0732).
Benlysta demonstrated improvement in fatigue compared with placebo measured by the FACIT-Fatigue Scale. The adjusted mean change of score at Week 52 from baseline is significantly greater with Benlysta compared to placebo (4.4 vs. 2.7, p=0.0130).
Subgroup analysis of the primary endpoint demonstrated that the greatest benefit was observed in patients with higher disease activity at baseline including patients with SELENA SLEDAI scores ≥10 or patients requiring steroids to control their disease or patients with low complement levels.
An additional, previously identified serologically active group, those patients with low complement and positive anti-dsDNA at baseline, also demonstrated a greater relative response, see Table 2 for results of this example of a higher disease activity group.
Table 2. Patients with low complement and positive anti-dsDNA at baseline:
| Subgroup | Anti-dsDNA positive AND low complement | |
|---|---|---|
| Placebo | Benlysta 200 mg weekly | |
SRI response rate at Week 521 (%) Observed treatment difference vs. placebo (%) | (n=108) 47.2 | (n=246) 64.6 (p=0.0014) 17.41 |
| Severe flares over 52 weeks: Patients experiencing a severe flare (%) Observed treatment difference vs. placebo (%) Time to severe flare [Hazard ratio (95% CI)] | (n=108) 31.5 | (n=248) 14.1 17.4 0.38 (0.24, 0.61) (p<0.0001) |
Prednisone reduction by ≥25% from baseline to ≤ 7.5 mg/day during weeks 24 through 522 (%) Observed treatment difference vs. placebo (%) | (n=70) 11.4 | (n=164) 20.7 (p=0.0844) 9.3 |
FACIT-fatigue score improvement from baseline at Week 52 (mean): Observed treatment difference vs. placebo (median difference) | (n=108) 2.4 | (n=248) 4.6 (p=0.0324) 2.1 |
1 Analysis of SRI response rate at Week 52 excluded any subject missing a baseline assessment (2 for Benlysta).
2 Among patients with baseline prednisone dose >7.5 mg/day.
The efficacy and safety of Benlysta in combination with a single cycle of rituximab have been studied in a Phase III, randomised, double-blind, placebo-controlled 104-week study including 292 patients (BLISSBELIEVE). The primary endpoint was the proportion of subjects with a state of disease control defined as a SLEDAI-2K score ≤2, achieved without immunosuppressants and with corticosteroids at a prednisone equivalent dose of ≤5 mg/day at Week 52. This was achieved in 19.4% (n=28/144) of the patients treated with Benlysta in combination with rituximab and in 16.7% (n=12/72) of the patients treated with Benlysta in combination with placebo (odds ratio 1.27; 95% CI: 0.60, 2.71; p=0.5342). A higher frequency of adverse events (91.7% vs. 87.5%), serious adverse events (22.2% vs. 13.9%) and serious infections (9.0% vs. 2.8%) were observed in patients treated with Benlysta in combination with rituximab as compared to Benlysta in combination with placebo.
Lupus nephritis
Subcutaneous injection
The efficacy and safety of Benlysta 200 mg administered subcutaneously to patients with active lupus nephritis is based on data from administration of Benlysta 10 mg/kg body weight intravenously and pharmacokinetic modelling and simulation (see section 5.2).
In the subcutaneous SLE study, described above, patients who had severe active lupus nephritis were excluded; however, 12% of patients had renal organ domain involvement at baseline (based on SELENA SLEDAI assessment). The following study in active lupus nephritis has been conducted.
Intravenous infusion
The efficacy and safety of Benlysta 10 mg/kg administered intravenously over a 1-hour period on Days 0, 14, 28, and then every 28 days, were evaluated in a 104-week randomised (1:1), double-blind, placebo-controlled, Phase III study (BEL114054) in 448 patients with active lupus nephritis. The patients had a clinical diagnosis of SLE according to ACR classification criteria, biopsy proven lupus nephritis Class III, IV, and/or V and had active renal disease at screening requiring standard therapy. Standard therapy included corticosteroids, 0 to 3 intravenous administrations of methylprednisolone (500 to1000 mg per administration), followed by oral prednisone 0.5 to1 mg/kg/day with a total daily dose ≤60 mg/day and tapered to ≤10 mg/day by Week 24, with:
- mycophenolate mofetil 1 to 3 g/day orally or mycophenolate sodium 720 to 2160 mg/day orally for induction and maintenance, or
- cyclophosphamide 500 mg intravenously every 2 weeks for 6 infusions for induction followed by azathioprine orally at a target dose of 2 mg/kg/day for maintenance.
This study was conducted in Asia, North America, South America, and Europe. Patient median age was 31 years (range: 18 to 77 years); the majority (88%) were female.
The primary efficacy endpoint was Primary Efficacy Renal Response (PERR) at Week 104 defined as a response at Week 100 confirmed by a repeat measurement at Week 104 of the following parameters: urinary protein:creatinine ratio (uPCR) ≤700 mg/g (79.5 mg/mmol) and estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m² or no decrease in eGFR of >20% from pre-flare value.
The primary efficacy endpoint was Primary Efficacy Renal Response (PERR) at Week 104 defined as a response at Week 100 confirmed by a repeat measurement at Week 104 of the following parameters: urinary protein:creatinine ratio (uPCR) ≤700 mg/g (79.5 mg/mmol) and estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m² or no decrease in eGFR of >20% from pre-flare value.
The major secondary endpoints included:
- Complete Renal Response (CRR) defined as a response at Week 100 confirmed by a repeat measurement at Week 104 of the following parameters: uPCR <500 mg/g (56.8 mg/mmol) and eGFR ≥90 mL/min/1.73 m² or no decrease in eGFR of >10% from pre-flare value.
- PERR at Week 52.
- Time to renal-related event or death (renal-related event defined as first event of end-stage renal disease, doubling of serum creatinine, renal worsening [defined as increased proteinuria, and/or impaired renal function], or receipt of renal disease-related prohibited therapy).
For PERR and CRR endpoints, steroid treatment had to be reduced to ≤10 mg/day from Week 24 to be considered a responder. For these endpoints, patients who discontinued treatment early, received prohibited medication, or withdrew from the study early were considered non-responders.
The proportion of patients achieving PERR at Week 104 was significantly higher in patients receiving Benlysta compared with placebo. The major secondary endpoints also showed significant improvement with Benlysta compared with placebo (Table 3).
Table 3. Efficacy results in adult patients with lupus nephritis:
| Efficacy endpoint | Placebo (n=223) | Benlysta 10 mg/kg (n=223) | Observed difference vs. placebo | Odds/Hazard ratio vs. placebo (95% CI) | P-value |
|---|---|---|---|---|---|
| PERR at Week 1041 Responders | 32.3% | 43.0% | 10.8% | OR 1.55 (1.04, 2.32) | 0.0311 |
| Components of PERR | |||||
| Urine protein:creatinine ratio ≤700 mg/g (79.5 mg/mmol) | 33.6% | 44.4% | 10.8% | OR 1.54 (1.04, 2.29) | 0.0320 |
| eGFR ≥60 mL/min/1.73 m² or no decrease in eGFR from pre-flare value of >20% | 50.2% | 57.4% | 7.2% | OR 1.32 (0.90, 1.94) | 0.1599 |
| Not treatment failure3 | 74.4% | 83.0% | 8.5% | OR 1.65 (1.03, 2.63) | 0.0364 |
| CRR at Week 1041 Responders | 19.7% | 30.0% | 10.3% | OR 1.74 (1.11, 2.74) | 0.0167 |
| Components of CRR | |||||
| Urine protein:creatinine ratio <500 mg/g (56.8 mg/mmol) | 28.7% | 39.5% | 10.8% | OR 1.58 (1.05, 2.38) | 0.0268 |
| eGFR ≥90 mL/min/1.73 m² or no decrease in eGFR from pre-flare value of >10% | 39.9% | 46.6% | 6.7% | OR 1.33 (0.90, 1.96) | 0.1539 |
| Not treatment failure3 | 74.4% | 83.0% | 8.5% | OR 1.65 (1.03, 2.63) | 0.0364 |
| PERR at Week 521 Responders | 35.4% | 46.6% | 11.2% | OR 1.59 (1.06, 2.38) | 0.0245 |
| Time to renal-related event or death1 Percentage of patients with event2 Time to event [Hazard ratio (95% CI)] | 28.3% | 15.7% | - - | HR 0.51 (0.34, 0.77) | 0.0014 |
1 PERR at Week 104 was the primary efficacy analysis; CRR at Week 104, PERR at Week 52 and time to renal-related event or death were included in the pre-specified testing hierarchy.
2 When excluding deaths from the analysis (1 for Benlysta; 2 for placebo), the percentage of patients with a renal-related event was 15.2% for Benlysta compared with 27.4% for placebo (HR = 0.51; 95% CI: 0.34, 0.78).
3 Treatment failure: Patients who took protocol-prohibited medication.
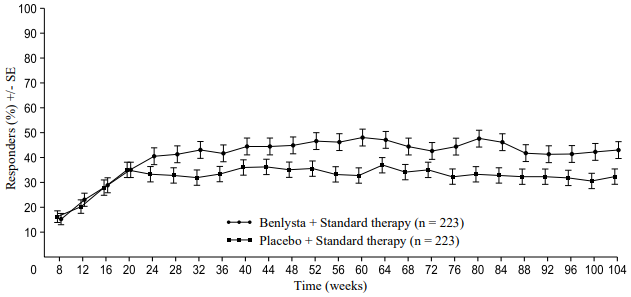
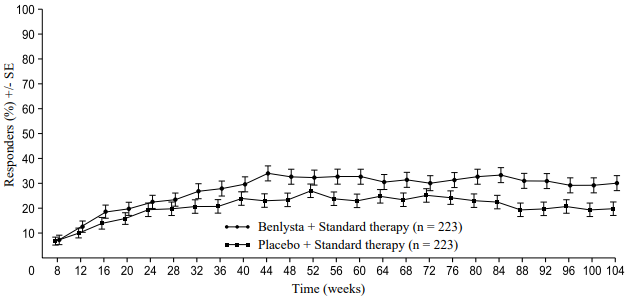
A numerically greater percentage of patients receiving Benlysta achieved PERR beginning at Week 24 compared with placebo, and this treatment difference was maintained through to Week 104. Beginning at Week 12, a numerically greater percentage of patients receiving Benlysta achieved CRR compared with placebo and the numerical difference was maintained through to Week 104 (Figure 2).
Figure 2. Response rates in adults with lupus nephritis by visit:
Primary Efficacy Renal Response (PERR)
Complete Renal Response (CRR)
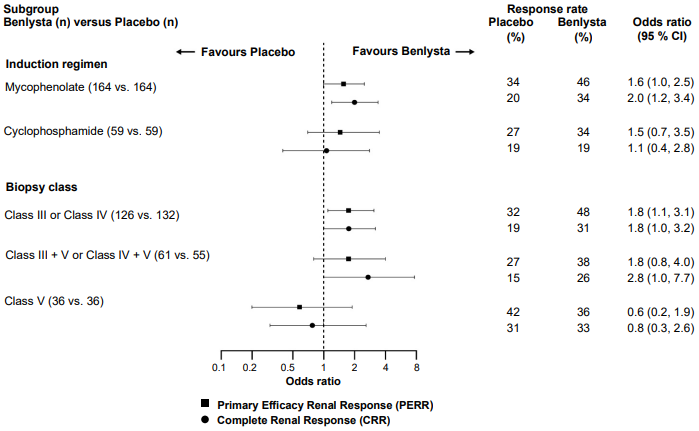
In descriptive subgroup analyses, key efficacy endpoints (PERR and CRR) were examined by induction regimen (mycophenolate or cyclophosphamide) and biopsy class (Class III or IV, Class III + V or Class IV + V, or Class V) (Figure 3).
Figure 3. Odds ratio of PERR and CRR at Week 104 across subgroups:
Age and race
There were no observed differences in efficacy or safety in SLE patients ≥65 years who received Benlysta intravenously or subcutaneously compared to the overall population in placebo-controlled studies; however, the number of patients aged ≥65 years (62 patients for efficacy and 219 for safety) is not sufficient to determine whether they respond differently to younger patients.
There were too few black patients enrolled in the placebo-controlled studies with subcutaneous Benlysta to draw meaningful conclusions about the effects of race on clinical outcomes.
The safety and efficacy of Benlysta administered intravenously have been studied in black patients. The currently available data are described in the Summary of Product Characteristics of Benlysta 120 mg and 400 mg powder for concentrate for solution for infusion.
Paediatric population
SLE
Subcutaneous injection
The safety and efficacy of Benlysta administered subcutaneously to paediatric patients 5 to <18 years of age with active SLE is supported by a population pharmacokinetic model and simulation integrating data from an open-label pharmacokinetic study of 25 paediatric patients with active SLE administered Benlysta subcutaneously (200908), and a study of paediatric patients with active SLE administered Benlysta intravenously (PLUTO) described below (see section 5.2).
Intravenous infusion
The safety and efficacy of Benlysta was evaluated in a randomised, double-blind, placebo-controlled, 52-week study (PLUTO) in 93 paediatric patients with a clinical diagnosis of SLE according to the ACR classification criteria. Patients had active SLE disease, defined as a SELENA-SLEDAI score ≥6 and positive autoantibodies at screening as described in the adult trials. Patients were on a stable SLE treatment regimen (standard of care) and had similar inclusion criteria as the adult studies. Patients who had severe active lupus nephritis, severe active CNS lupus, primary immunodeficiency, IgA deficiency or acute or chronic infections requiring management were excluded from the study. The study was conducted in the US, South America, Europe, and Asia. Patient median age was 15 years (range 6 to 17 years). In the 5- to 11-year-old-group (n=13) the SELENA-SLEDAI score ranged from 4 to 13, and in 12- to 17-year-old-group (n=79) the SELENA-SLEDAI score ranged from 4 to 20. The majority (94.6 %) of patients were female. The study was not powered for any statistical comparisons and all data are descriptive.
The primary efficacy endpoint was the SLE Responder Index (SRI) at Week 52 as described in the adult intravenous trials. There was a higher proportion of paediatric patients achieving an SRI response in patients receiving Benlysta compared with placebo. The response for the individual components of the endpoint were consistent with that of the SRI (Table 4).
Table 4. Paediatric response rate at Week 52:
| Response1 | Placebo (n=40) | Benlysta 10 mg/kg (n=53) |
|---|---|---|
| SLE Responder Index (%) Odds ratio (95% CI) vs. placebo | 43.6 (17/39) | 52.8 (28/53) 1.49 (0.64, 3.46) |
| Components of SLE Responder Index | ||
| Percent of patients with reduction in SELENA-SLEDAI ≥4 (%) Odds ratio (95% CI) vs. placebo | 43.6 (17/39) | 54.7 (29/53) 1.62 (0.69, 3.78) |
| Percent of patients with no worsening by BILAG index (%) Odds ratio (95% CI) vs. placebo | 61.5 (24/39) | 73.6 (39/53) 1.96 (0.77, 4.97) |
| Percent of patients with no worsening by PGA (%) Odds ratio (95% CI) vs. placebo | 66.7 (26/39) | 75.5 (40/53) 1.70 (0.66, 4.39) |
1 Analyses excluded any subject missing a baseline assessment for any of the components (1 for placebo).
Among patients experiencing a severe flare, the median study day of the first severe flare was Day 150 in the Benlysta group and Day 113 in the placebo group. Severe flares were observed in 17.0% of the Benlysta group compared to 35.0% of the placebo group over the 52 weeks of observation (observed treatment difference = 18.0%; hazard ratio = 0.36, 95% CI: 0.15, 0.86). This was consistent with the findings from the adult intravenous clinical trials.
Using the Paediatric Rheumatology International Trials Organisation/American College of Rheumatology (PRINTO/ACR) Juvenile SLE Response Evaluation Criteria, a higher proportion of paediatric patients receiving Benlysta demonstrated improvement compared with placebo (Table 5).
Table 5. PRINTO/ACR response rate at Week 52:
| Proportion of patients with at least 50% improvement in any 2 of 5 components1 and no more than one of the remaining worsening by more than 30% | Proportion of patients with at least 30% improvement in 3 of 5 components1 and no more than one of the remaining worsening more than 30% | |||
|---|---|---|---|---|
| Placebo n=40 | Benlysta 10 mg/kg n=53 | Placebo n=40 | Benlysta 10 mg/kg n=53 | |
| Response, n (%) | 14/40 (35.0) | 32/53 (60.4) | 11/40 (27.5) | 28/53 (52.8) |
| Observed difference vs. Placebo | 25.38 | 25.33 | ||
| Odds ratio (95% CI) vs. Placebo | 2.74 (1.15, 6.54) | 2.92 (1.19, 7.17) | ||
1 The five PRINTO/ACR components were percent change at Week 52 in: Parent's Global Assessment (Parent GA), PGA, SELENA SLEDAI score, 24-hour proteinuria, and, Paediatric Quality of Life Inventory – Generic Core Scale (PedsQL GC) physical functioning domain score.
Pharmacokinetic properties
The subcutaneous pharmacokinetic parameters below are based on population parameter estimates from 661 subjects, comprised of 554 SLE patients and 107 healthy subjects, who received Benlysta subcutaneously.
Absorption
Benlysta in the pre-filled pen is administered by subcutaneous injection.
Following subcutaneous administration the bioavailability of belimumab was approximately 74%. Steady-state exposure was reached after approximately 11 weeks of subcutaneous administration. The maximum serum concentration (Cmax) of belimumab at steady state was 108 μg/ml.
Distribution
Belimumab was distributed to tissues with steady-state volume (Vss) of distribution of approximately 5 litres.
Biotransformation
Belimumab is a protein for which the expected metabolic pathway is degradation to small peptides and individual amino acids by widely distributed proteolytic enzymes. Classical biotransformation studies have not been conducted.
Elimination
Following subcutaneous administration, belimumab had a terminal half-life of 18.3 days. The systemic clearance was 204 ml/day.
Lupus nephritis study
A population pharmacokinetic analysis was conducted in 224 adult patients with lupus nephritis who received Benlysta 10 mg/kg intravenously (Days 0, 14, 28, and then every 28 days up to 104 weeks). In patients with lupus nephritis, due to renal disease activity, belimumab clearance was initially higher than observed in SLE studies; however, after 24 weeks of treatment and throughout the remainder of the study, belimumab clearance and exposure were similar to that observed in adult patients with SLE who received belimumab 10 mg/kg body weight intravenously.
Based on population pharmacokinetic modelling and simulation, the steady-state average concentrations of subcutaneous administration of belimumab 200 mg once weekly in adults with lupus nephritis are predicted to be similar to those observed in adults with lupus nephritis receiving belimumab 10 mg/kg body weight intravenously every 4 weeks.
Special patient populations
Paediatric population
The pharmacokinetic parameters of belimumab administered subcutaneously are based on a population pharmacokinetic analysis of 25 patients from a Phase II pharmacokinetic study in paediatric patients with SLE receiving belimumab subcutaneously and the Phase II study in paediatric patients with SLE receiving belimumab intravenously. Following subcutaneous administration of 200 mg of belimumab in paediatric patients 5 to less than 18 years of age [weekly (patients weighing ≥50 kg), every 10 days (patients weighing 30 to <50 kg) or every 2 weeks (patients weighing 15 to <30 kg)], the steady state average belimumab concentration is estimated to be similar to that of adult SLE subjects following subcutaneous administration of 200 mg belimumab weekly, and similar to that of paediatric SLE subjects following intravenous administration of belimumab 10 mg/kg body weight on Days 0, 14 and 28, and at 4-week intervals thereafter. Simulated steady-state geometric mean Cmax, Cavg, Cmin, and AUC (calculated over the dosing interval) are estimated to be 124 μg/mL, 119 μg/mL, 111 μg/mL and 834 day•μg/mL for paediatric patients weighing ≥50 kg receiving belimumab once weekly, 114 μg/mL, 105 μg/mL, 91 μg/mL and 1051 day•μg/mL for paediatric patients weighing 30 to <50 kg receiving belimumab every 10 days, and 119 μg/mL, 103 μg/mL, 79 μg/mL and 1438 day•μg/mL for paediatric patients weighing 15 to <30 kg receiving belimumab every 2 weeks.
Elderly
Benlysta has been studied in a limited number of elderly patients. Age did not affect belimumab exposure in the subcutaneous population pharmacokinetic analysis. However, given the small number of subjects ≥65, an effect of age cannot be ruled out conclusively.
Renal impairment
No specific studies have been conducted to examine the effects of renal impairment on the pharmacokinetics of belimumab. During clinical development, Benlysta was studied in a limited number of SLE patients with mild (creatinine clearance [CrCl] ≥60 and <90 ml/min), moderate (CrCl ≥30 and <60 ml/min), or severe (CrCl ≥15 and <30 ml/min) renal impairment: 121 patients with mild renal impairment and 30 patients with moderate renal impairment received Benlysta subcutaneously; 770 patients with mild renal impairment, 261 patients with moderate renal impairment and 14 patients with severe renal impairment received Benlysta intravenously.
No clinically significant reduction in systemic clearance as a result of renal impairment was observed. Therefore, no dose adjustment is recommended for patients with renal impairment.
Hepatic impairment
No specific studies have been conducted to examine the effects of hepatic impairment on the pharmacokinetics of belimumab. IgG1 molecules such as belimumab are catabolised by widely distributed proteolytic enzymes, which are not restricted to hepatic tissue and changes in hepatic function are unlikely to have any effect on the elimination of belimumab.
Body weight/Body mass index (BMI)
The effects of body weight and BMI on belimumab exposure after subcutaneous administration in adults were not considered clinically meaningful. There was no significant impact on efficacy and safety based on weight. Therefore, no dose adjustment in adults is recommended.
The effects of body weight on belimumab exposure after subcutaneous administration in paediatric patients have been determined using a population pharmacokinetic model. Paediatric patients with lower body weight have lower belimumab clearance and volume of distribution resulting in increased exposure. To ensure belimumab exposures remain within acceptable limits and are consistent across the paediatric weight range, patients with lower body weight are dosed belimumab less frequently (see section 4.2).
Transitioning from intravenous to subcutaneous administration
SLE
Patients with SLE transitioning from 10 mg/kg body weight intravenously every 4 weeks to a 200 mg subcutaneous regimen using a 1 to 4 week switching interval had pre-dose belimumab serum concentrations at their first subcutaneous dose close to their eventual subcutaneous steady-state trough concentration (see section 4.2). Based on simulations with population pharmacokinetic parameters, the steady-state average belimumab concentrations for 200 mg subcutaneous every week (in adult patients, and in paediatric patients 5 to under 18 years of age and ≥50 kg), every 10 days (in paediatric patients 5 to under 18 years of age and 30 to <50 kg) or every 2 weeks (in paediatric patients 5 to under 18 years of age and 15 to <30 kg), were similar to 10 mg/kg body weight intravenous every 4 weeks.
Lupus nephritis
One to 2 weeks after completing the first 2 intravenous doses, patients with lupus nephritis transitioning from 10 mg/kg body weight intravenously to 200 mg subcutaneously weekly, are predicted to have average belimumab serum concentrations similar to patients dosed with 10 mg/kg body weight intravenously every 4 weeks based on population pharmacokinetic simulations (see section 4.2).
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on studies of repeated dose toxicity and toxicity to reproduction.
Intravenous and subcutaneous administration to monkeys resulted in the expected reduction in the number of peripheral and lymphoid tissue B cell counts with no associated toxicological findings.
Reproductive studies have been performed in pregnant cynomolgus monkeys receiving belimumab 150 mg/kg body weight by intravenous infusion (approximately 9 times the anticipated maximum human clinical exposure) every 2 weeks for up to 21 weeks, and belimumab treatment was not associated with direct or indirect harmful effects with respect to maternal toxicity, developmental toxicity, or teratogenicity.
Treatment-related findings were limited to the expected reversible reduction of B cells in both dams and infants and reversible reduction of IgM in infant monkeys. B cell numbers recovered after the cessation of belimumab treatment by about 1 year post-partum in adult monkeys and by 3 months of life in infant monkeys; IgM levels in infants exposed to belimumab in utero recovered by 6 months of age.
Effects on male and female fertility in monkeys were assessed in the 6-month repeat dose toxicology studies of belimumab at doses up to and including 50 mg/kg body weight. No treatment-related changes were noted in the male and female reproductive organs of sexually mature animals. An informal assessment of menstrual cycling in females demonstrated no belimumab-related changes.
As belimumab is a monoclonal antibody no genotoxicity studies have been conducted. No carcinogenicity studies or fertility studies (male or female) have been performed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.