BERODUAL RESPIMAT Inhalation solution Ref.[50758] Active ingredients: Ipratropium
Source: Z-Index G-Standaard (NL) Revision Year: 2022 Publisher: Boehringer Ingelheim International GmbH, Binger Straße 173, D-55216 Ingelheim am Rhein, Germany
4.1. Therapeutic indications
Berodual Respimat is indicated for the prevention and treatment of bronchospasm in asthma and chronic obstructive pulmonary disease (COPD).
Concomitant anti-inflammatory therapy should be considered.
4.2. Posology and method of administration
Posology
The dosage should be adapted to the individual requirements. The following dosages are recommended for adults.
Acute asthma episodes
One actuation of Berodual Respimat is sufficient for prompt relief in many cases. In more severe cases, if breathing has not noticeably improved after 5 minutes, one further actuation may be taken. If an attack has not been relieved by 2 actuations, further actuations may be required. In these cases, patients should be advised to consult the doctor or the nearest hospital immediately.
Intermittent and long-term treatment (in asthma Berodual Respimat should be used only on an asneeded basis)
Adults: 1 actuation per administation of Berodual Respimat up to 4 times a day.
The total daily dose should not exceed 6 actuations, because generally a higher dose is not likely to provide increased efficacy. However, the risk of potentially serious adverse reaction may be increased.
Paediatric population: Berodual Respimat is not recommended for use in children below 18 years due to insufficient data on safety and efficacy.
Method of administration
This medicinal product is intended for inhalation use only. The cartridge can only be inserted and used in the Respimat device. Respimat is an inhaler device that generates a spray for inhalation. It is a single patient device intended for multiple uses.
Patients should read the instructions on How to use the Respimat inhaler device before they start using Berodual Respimat. To ensure proper administration of the medicinal product, the patient should be shown how to use the inhaler by a physician or other health care professional.
Instructions for handling and use of the Respimat inhaler
- If Berodual Respimat has not been used for more than 7 days release one puff towards the ground.
- If Berodual Respimat has not been used for more than 21 days repeat steps 4 to 6 under ‘Prepare for first use’ until a cloud is visible. Then repeat steps 4 to 6 three more times
- Do not touch the piercing element inside the clear base.
How to care for Berodual Respimat
Clean the mouthpiece including the metal part inside the mouthpiece with a damp cloth or tissue only, at least once a week.
Any minor discoloration in the mouthpiece does not affect Berodual Respimat inhaler performance.
If necessary, wipe the outside of Berodual Respimat inhaler with a damp cloth.
When to get a new Berodual Respimat
- Berodual Respimat inhaler contains 120 puffs (120 doses) if used as indicated.
- The dose indicator shows approximately how much medication is left.
- When the dose indicator enters the red area of the scale the patient needs to get a new prescription; there is approximately medication for 7 days left (28 puffs).
- Once the dose indicator reaches the end of the red scale, BERODUAL RESPIMAT locks automatically – no more doses can be released. At this point, the clear base cannot be turned any further.
- Berodual Respimat should be discarded three months after the patient has prepared it for first use, even if it has not been fully used or used at all.
Prepare for first use
| 1. Remove clear base Keep the cap closed. Press the safety catch while firmly pulling off the clear base with the other hand. | 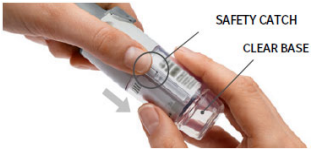 |
| 2. Insert cartridge Insert the narrow end of the cartridge into the inhaler. Place the inhaler on a firm surface and push down firmly until it clicks into place. Do not remove the cartridge once it has been inserted into the inhaler. |  |
| 3. Replace clear base Put the clear base back into place until it clicks. Do not remove the clear base again. |  |
| 4. Turn Keep the cap closed. Turn the clear base in the direction of the arrows on the label until it clicks (half a turn). |  |
| 5. Open Open the cap until it snaps fully open |  |
| 6. Press Point the inhaler toward the ground Press the dose-release button. Close the cap. Repeat steps 4-6 until a cloud is visible. After a cloud is visible, repeat steps 4-6 three more times. The inhaler is now ready to use. These steps will not affect the number of doses available. After preparation the inhaler will be able to deliver 120 puffs (120 doses). |  |
Daily use
| TURN Keep the cap closed. TURN the clear base in the direction of the arrows on the label until it clicks (half a turn). |  |
| OPEN OPEN the cap until it snaps fully open. | 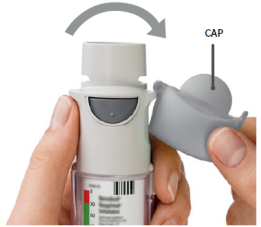 |
| PRESS Breathe out slowly and fully. Close the lips around the mouthpiece without covering the air vents. Point the Inhaler to the back of the throat. While taking a slow, deep breath through the mouth, PRESS the dose-release button and continue to breathe in slowly for as long as comfortable. Hold the breath for 10 seconds or for as long as comfortable. Close the cap until the inhaler is used again. | 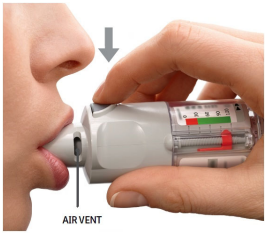 |
4.9. Overdose
Symptoms
The effects of overdose are expected to be primarily related to fenoterol.
The expected symptoms with overdose are those of excessive ß-adrenergic stimulation, the most prominent being tachycardia, palpitation, tremor, hypertension, hypotension, widening of the pulse pressure, anginal pain, arrhythmias, and flushing.
Metabolic acidosis has also been observed with fenoterol when applied in doses higher than recommended for the approved indications of Berodual. Hypokalaemia may occur following overdose with fenoterol. Serum potassium levels should be monitored.
Expected symptoms of overdose with ipratropium bromide (such as dry mouth, visual accommodation disorder, increase of heart rate) are mild and because the systemic bioavailability of inhaled ipratropium is very low.
Treatment of overdose
Treatment with Berodual Respimat should be discontinued. Acid base and electrolyte monitoring should be considered.
Administration of sedatives, tranquilisers; in severe cases intensive care treatment. Beta-receptor blockers, preferably beta1-selective, may be used as specific antidotes; however, a possible increase in bronchial obstruction must be taken into account and the dose should be adjusted carefully in patients suffering from asthma or COPD because of the risk of precipitating severe bronchospasm, which may be fatal.
6.3. Shelf life
3 years.
This includes a 3 months in-use period. The cartridge has an in-use shelf life of 3 months after insertion in the Respimat.
6.4. Special precautions for storage
Do not freeze.
6.5. Nature and contents of container
Type and material of the container in contact with the medicinal product:
Solution filled into a 4.5 ml polyethylene/polypropylene cartridge with a polypropylene cap with integrated silicone sealing ring. The cartridge is enclosed within an aluminium cylinder.
Pack sizes and devices supplied:
- Original package: 1 Respimat inhaler and one 4.5 ml cartridge, delivering 120 metered doses.
- Double package: 2 single packages, each containing 1 Respimat inhaler and one 4.5 ml cartridge, each delivering 120 metered doses.
- Hospital package: 8 single packages, each containing 1 Respimat inhaler and one 4.5 ml cartridge, each delivering 120 metered doses.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.

