BEVESPI AEROSPHERE Pressurised inhalation, suspension Ref.[51295] Active ingredients: Eformoterol Formoterol and Glycopyrronium bromide Glycopyrronium
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: AstraZeneca AB, SE-151 85 Södertälje, Sweden
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for obstructive airway diseases, adrenergics in combination with anticholinergics
ATC code: R03AL07
Mechanism of action
Bevespi Aerosphere contains two bronchodilators: glycopyrronium, a long-acting muscarinic antagonist (also referred to as an anticholinergic), and formoterol, a long-acting β2-adrenergic agonist with a rapid onset of action.
Glycopyrronium has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of the M3 receptor at the smooth muscle leading to bronchodilation. Formoterol causes direct relaxation of airway smooth muscle as a consequence of the increase in cyclic AMP through activation of adenylate cyclase. The combination of these substances with different mechanisms of action results in additive efficacy compared to use with either component alone.
As a consequence of the differential density of muscarinic receptors and β2-adrenoceptors in the central and peripheral airways of the lung, muscarinic antagonists are more effective in relaxing central airways, and β2-adrenergic agonists are more effective in relaxing peripheral airways; relaxation of both central and peripheral airways with combination treatment may contribute to its beneficial effects on lung function.
Pharmacodynamic effects
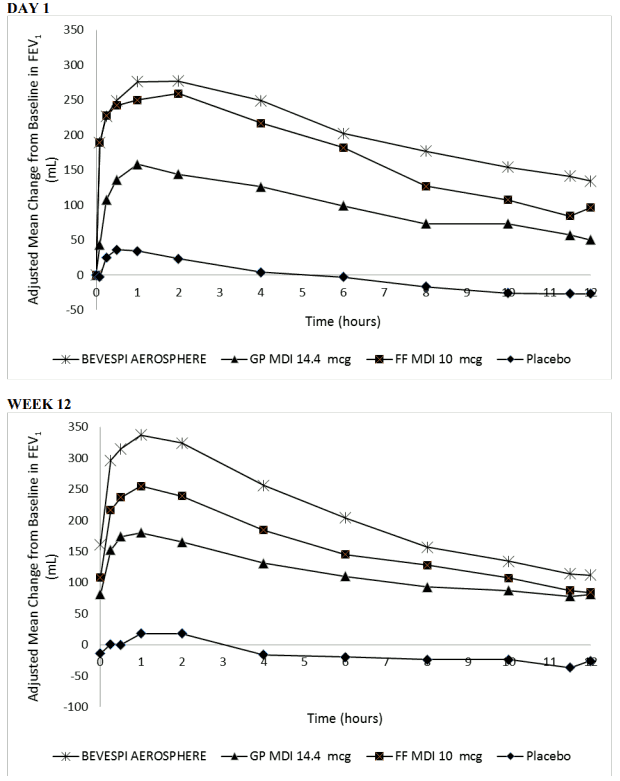
In three Phase III, 24-week studies (PINNACLE 1, PINNACLE 2 and PINNACLE 4) Bevespi Aerosphere provided improvements over placebo in lung function (as measured by morning pre-dose trough forced expiratory volume in 1 second [FEV1]), with a demonstrated onset of action at 5 minutes following administration of the first dose on Day 1 (improvement over placebo by 187 mL, 186 mL and 179 mL in PINNACLE 1, PINNACLE 2 and PINNACLE 4, respectively [p<0.001]). The mean bronchodilator effect derived from serial FEV1 measurements at Day 1 and Week 12 from PINNACLE 1 are shown in Figure 1. In PINNACLE 2, the results were similar to those observed in PINNACLE 1.
Figure 1. Mean Change from Baseline in FEV1 over Time on Day 1 and at Week 12:
Cardiac electrophysiology
A placebo- and active-controlled (moxifloxacin) thorough QT study in 69 healthy subjects did not demonstrate a clinically relevant effect on the QT interval, using a threshold of 10 ms. The largest mean (90% upper confidence bound) differences from placebo in baseline- and individually corrected QT was 3.1 (4.7) ms for Bevespi Aerosphere (14.4/10 micrograms) and 7.6 (9.2) ms for glycopyrronium/formoterol with eight times the recommended dose of glycopyrronium and four times the recommended dose of formoterol.
Clinical efficacy
The clinical development program for Bevespi Aerosphere included three 24-week, randomised, double-blind, placebo-controlled, parallel-group pivotal Phase III studies in 5,433 patients with moderate to very severe COPD (PINNACLE 1, PINNACLE 2 and PINNACLE 4).
Effects on lung function
In studies PINNACLE 1, PINNACLE 2 and PINNACLE 4, Bevespi Aerosphere showed improvements in trough FEV1 over 24 weeks relative to placebo, glycopyrronium and formoterol (p<0.0001) [see Table 2]. There was no attenuation of the bronchodilator effect over time. Bevespi Aerosphere also showed improvements in peak FEV1 within 2 hours post-dose over 24 weeks relative to placebo, glycopyrronium and formoterol (p<0.0001) [see Table 2].
There were improvements in trough FEV1 irrespective of age, sex, degree of airflow limitation, baseline symptoms, smoking status, or inhaled corticosteroid use.
Symptomatic outcomes
Breathlessness
In PINNACLE 1 and PINNACLE 2, Bevespi Aerosphere provided improvements in breathlessness as demonstrated by Self-administered Computerised Transitional Dyspnoea Index (SAC TDI) focal score over 24 weeks compared to placebo and glycopyrronium (see Table 2). Improvements compared to formoterol were observed in PINNACLE 2 (see Table 2). In PINNACLE 4, Bevespi Aerosphere provided improvements in breathlessness as demonstrated by TDI focal score over 24 weeks compared to placebo and glycopyrronium (see Table 2).
Health-related quality of life
In PINNACLE 1, PINNACLE 2 and PINNACLE 4, Bevespi Aerosphere provided an improvement in disease-specific health-related quality of life, as indicated by a reduction in the St George's Respiratory Questionnaire (SGRQ) total score over 24 weeks compared to placebo and glycopyrronium (see Table 2). There were improvements compared to formoterol in PINNACLE 1 and PINNACLE 2.
Table 2. Lung function, symptomatic and health related quality of life outcomes over 24 weeks:
| Treatment comparisons with Bevespi Aersophere | Treatment difference (95% confidence intervals, p-value) | ||||
|---|---|---|---|---|---|
| Trough FEV1 (ml)a | Peak FEV1 (ml) | SAC-TDI / TDI Focal Scoreb | SGRQ total score | Daily rescue Ventolin (inhalations/day)c | |
| PINNACLE 1 | |||||
| Bevespi Aerosphere (N=526) vs placebo (N=219) | 158 (132, 183) p<0.0001 | 288 (259, 317) p<0.0001# | 0.47 (0.21, 0.72) p=0.0003 | -2.39 (-4.07, -0.71) p=0.0053# | -1.08 (-1.43, -0.73) p<0.0001# |
| Bevespi Aerosphere (N=526) vs Glycopyrronium (N=451) | 60 (39, 80) p<0.0001 | 123 (100, 146) p<0.0001# | 0.27 (0.07, 0.47) p=0.0086# | -1.90 (-3.24, 0.57) p=0.0052# | -0.26 (-0.53, 0.01) p=0.0619 |
| Bevespi Aerosphere (N=526) vs formoterol fumarate (N=449) | 64 (44, 84) p<0.0001 | 81 (59, 104) p<0.0001# | 0.16 (-0.03, 0.36) p=0.1060 | -0.75 (-2.08, 0.57) p=0.2640 | -0.01 (-0.27, 0.26) p=0.9683 |
| PINNACLE 2 | |||||
| Bevespi Aerosphere (N=510) vs placebo (N=223) | 129 (103, 155) p<0.0001 | 278 (249, 308) p<0.0001 | 0.33 (0.11, 0.56) p=0.0041 | -1.66 (-3.34, 0.02) p=0.0534 | -1.04 (-1.37, -0.72) p<0.0001 |
| Bevespi Aerosphere (N=510) vs Glycopyrronium (N=439) | 55 (34, 76) p<0.0001 | 129 (106, 153) p<0.0001 | 0.21 (0.03, 0.40) p=0.0199 | -1.28 (-2.62, 0.06) p=0.0605 | -0.57 (-0.83, -0.31) p<0.0001 |
| Bevespi Aerosphere (N=510) vs formoterol fumarate (N=437) | 57 (36, 78) p<0.0001 | 76 (52, 99) p<0.0001 | 0.28 (0.10, 0.46) p=0.0028 | -1.22 (-2.56, 0.13) p=0.0760 | -0.29 (-0.55, -0.03) p=0.0274# |
| PINNACLE 4 | |||||
| Bevespi Aerosphere (N=551) vs placebo (N=235) | 155 (129, 180) p<0.0001 | 293 (265, 321) p<0.0001 | 0.80 (0.47, 1.13) p<0.0001 | -3.50 (-5.18, -1.82) p<0.0001 | -0.98 (-1.47, -0.49) p<0.0001 |
| Bevespi Aerosphere (N=551) vs glycopyrronium (N=474) | 55 (35, 76) p<0.0001 | 141 (119, 163) p<0.0001 | 0.33 (0.07, 0.59) p=0.0125 | -1.62 (-2.94, -0.30) p=0.0165 | -0.77 (-1.16, -0.38) p<0.0001 |
| Bevespi Aerosphere (N=551) vs formoterol fumarate (N=480) | 72 (52, 92) p<0.0001 | 97 (75, 119) p<0.0001 | 0.15 (-0.11, 0.41) p=0.2530 | -0.27 (-1.59, 1.05) p=0.6908 | -0.41 (-0.80, -0.03) p=0.0345# |
N number in Intent to Treat population
a primary endpoint in all studies
b PINNACLE 1 and PINNACLE 2 used SAC-TDI. PINNACLE 4 used TDI. SAC-TDI was a primary endpoint in PINNACLE 1 and PINNACLE 2 only
c From the Rescue Ventolin User Population in PINNACLE 4
# A hierarchical statistical testing procedure was used in this study and this comparison was below a comparison that did not achieve statistical significance. Therefore, statistical significance on this comparison cannot be inferred.
COPD exacerbations
The individual studies were not specifically designed to evaluate the effect of treatments on COPD exacerbations and patients were withdrawn from the studies if a severe exacerbation or more than 2 moderate exacerbations occurred.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Bevespi Aerosphere in all subsets of the paediatric population in COPD (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Following inhalation of the glycopyrronium and formoterol combination, the pharmacokinetics of each component was similar to those observed when each active substance was administered separately. For pharmacokinetic purposes, each component can therefore be considered separately.
Effect of a spacer
The use of Bevespi Aerosphere with the Aerochamber Plus Flow-Vu spacer in COPD patients increased the total systemic exposure to glycopyrronium (as measured by AUC0-12) by 16% while formoterol exposure was unchanged.
Absorption
Following inhaled administration of Bevespi Aerosphere in subjects with COPD, glycopyrronium Cmax occurred at approximately 5 minutes, and formoterol Cmax occurred within 20 to 60 minutes. Steady state is achieved within 2-3 days of repeated dosing of Bevespi Aerosphere, and the extent of exposure is approximately 2.3 times and 1.5 times higher than after the first dose, for glycopyrronium and formoterol, respectively.
A lung deposition study with Bevespi Aerosphere conducted in healthy volunteers demonstrated that on average 38% of the nominal dose is deposited into the lung. Both central and peripheral deposition were observed.
Distribution
Glycopyrronium
The estimated glycopyrronium Vc/F (volume of the central compartment), and Vp1/F (volume of the peripheral compartment) are 741 L, and 2 990 L, respectively, via population pharmacokinetic analysis. Over the concentration range of 2-500 nmol/L, plasma protein binding of glycopyrronium ranged from 43% to 54%.
Formoterol
The estimated formoterol Vc/F (volume of the central compartment), and Vp1/F (volume of the peripheral compartment) are 1 030 L, and 647 L, respectively, via population pharmacokinetic analysis. Over the concentration range of 10-500 nmol/L, plasma protein binding of formoterol ranged from 46% to 58%.
Biotransformation
Glycopyrronium
Based on literature, and an in-vitro human hepatocyte study, metabolism plays a minor role in the overall elimination of glycopyrronium. CYP2D6 was found to be the predominant enzyme involved in the metabolism of glycopyrronium.
In-vitro studies indicate the glycopyrronium does not inhibit any subtype of cytochrome P450 and that there is no induction of CYP1A2, 2B6, or 3A4.
Formoterol
The primary metabolism of formoterol is by direct glucuronidation and by O-demethylation followed by conjugation to inactive metabolites. Secondary metabolic pathways include deformylation and sulfate conjugation. CYP2D6 and CYP2C have been identified as being primarily responsible for Odemethylation.
In-vitro studies indicate that formoterol does not inhibit the CYP450 enzymes at therapeutically relevant concentrations.
Elimination
After intravenous administration of a 0.2 mg dose of radiolabelled glycopyrronium, 85% of the dose was recovered in urine 48 hours post dose and some of radioactivity was also recovered in bile. The terminal elimination half-life of glycopyrronium following oral inhalation derived via population pharmacokinetics analysis was 15 hours.
The excretion of formoterol was studied in six healthy subjects following simultaneous administration of radiolabelled formoterol via the oral and intravenous routes. In that study, 62% of the radiolabelled formoterol was excreted in the urine while 24% was eliminated in the faeces. The terminal elimination half-life of formoterol following oral inhalation derived via population pharmacokinetics analysis was 13 hours.
Linearity/non-linearity
Linear pharmacokinetics were observed for glycopyrronium (dose range: 14.4 to 115.2 mcg) and formoterol (dose range: 2.4 to 19.2 mcg) after oral inhalation.
Special populations
Elderly
Based on available data, no adjustment of the dose of Bevespi Aerosphere in geriatric patients is necessary.
Renal impairment
Studies evaluating the effect of renal impairment on the pharmacokinetics of glycopyrronium and formoterol have not been conducted. The effect of renal impairment on the exposure to glycopyrronium and formoterol for up to 12 weeks was evaluated in a population pharmacokinetic analysis. Estimated glomerular filtration rate (eGFR) varied from 30-196 mL/min, representing a range of moderate to no renal impairment. The systemic exposure (AUC0-12) in subjects with COPD with moderate-severe renal impairment (eGFR of 30-45 mL/min) is approximately 30% higher for glycopyrronium compared to subjects with COPD with normal renal function (eGFR of >90 mL/min). Subjects with COPD with both low body weight and moderate-severe impaired renal function may have an approximate doubling of systemic exposure to glycopyrronium. Renal function was found not to affect exposure to formoterol.
Hepatic impairment
No pharmacokinetic studies have been performed with Bevespi Aerosphere in patients with hepatic impairment. However, because formoterol is primarily eliminated via hepatic metabolism, an increased exposure can be expected in patients with severe liver impairment. Glycopyrronium is primarily cleared from the systemic circulation by renal excretion and hepatic impairment would therefore not be expected to lead to unsafe systemic exposure.
Other special populations
A population pharmacokinetic analysis of glycopyrronium was performed based on data collected in a total of 311 subjects with COPD. The pharmacokinetics of glycopyrronium was best described by a two-compartment disposition model with first-order absorption and linear elimination. The typical clearance (CL/F) of glycopyrronium was 124 L/h.
A population pharmacokinetic analysis of formoterol was performed based on data collected in a total of 437 subjects with COPD. The pharmacokinetics of formoterol was best described by a twocompartment disposition model with a first-order rate constant of absorption and linear elimination. The typical clearance (CL/F) of formoterol was 99 L/h.
Dose adjustments are not necessary based on the effect of age, sex and weight on the pharmacokinetic parameters of glycopyrronium and formoterol.
There were no major differences in total systemic exposure (AUC) for both compounds between healthy Japanese and Western subjects. Insufficient pharmacokinetic data are available to compare exposure for other ethnicities or races.
5.3. Preclinical safety data
Non-clinical data reveal no specific hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenic potential.
The toxicity observed in studies with the combination of glycopyrronium and formoterol in dogs were associated with the pharmacological actions of formoterol, including effects mainly on the cardiovascular system, consisting of hyperaemia, tachycardia, arrhythmias and myocardial lesions. These are known pharmacological manifestations seen after administration of high doses of β-adrenoceptor agonists. No significant effects attributable to glycopyrronium were seen.
Animal reproduction studies with formoterol have shown a slightly reduced fertility in male rats at high systemic exposure and implantation losses, as well as decreased early postnatal survival and birth weight at considerably higher systemic exposures than those reached during clinical use. However, these animal experimental results have little relevance to man. A slight increase in the incidence of uterine leiomyomas has been observed in rats and mice treated with formoterol; an effect which is considered to be a class-effect in rodents after long-term exposure to high doses of β2-adrenoreceptor agonists.
Animal reproduction studies with glycopyrronium have shown reduced rat and rabbit fetal weights, and low body weight gain of rat offspring before weaning was observed at considerably higher systemic exposures than those reached during clinical use. No evidence of carcinogenicity was seen in 2-year studies in rats and mice.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.