BEXSERO Suspension for injection Ref.[50804] Active ingredients: Neisseria meningitidis OMPC
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: GSK Vaccines S.r.l., Via Fiorentina 1, 53100 Siena, Italy
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: meningococcal vaccines
ATC code: J07AH09
Mechanism of action
Immunisation with Bexsero is intended to stimulate the production of bactericidal antibodies that recognize the vaccine antigens NHBA, NadA, fHbp, and PorA P1.4 (the immunodominant antigen present in the OMV component) and are expected to be protective against Invasive Meningococcal Disease (IMD). As these antigens are variably expressed by different strains, meningococci that express them at sufficient levels are susceptible to killing by vaccine-elicited antibodies. The Meningococcal Antigen Typing System (MATS) was developed to relate antigen profiles of different strains of meningococcal group B bacteria to killing of the strains in the serum bactericidal assay with human complement (hSBA). A survey of approximately 1 000 different invasive meningococcal group B isolates collected during 2007-2008 in 5 European countries showed that, depending on the country of origin, between 73% and 87% of meningococcal group B isolates had an appropriate MATS antigen profile to be covered by the vaccine. Overall, 78% (95% CI: 63-90) of the approximately 1 000 strains were potentially susceptible to vaccine-induced antibodies.
Clinical efficacy
The efficacy of Bexsero has not been evaluated through clinical trials. Vaccine efficacy has been inferred by demonstrating the induction of serum bactericidal antibody responses to each of the vaccine antigens (see section Immunogenicity). Vaccine effectiveness and impact have been demonstrated in real-world settings.
Impact of vaccination on disease incidence
In the UK, Bexsero was introduced into the national immunisation programme (NIP) in September 2015 using a two-dose schedule in infants (at 2 and 4 months of age) followed by a booster dose (at 12 months of age). In this context, Public Health England (PHE) conducted a 3-year observational study at the national level covering the entire birth cohort.
After three years of the programme, a statistically significant reduction of 75% [Incidence Rate Ratio (IRR) 0.25 (95% CI: 0.19-0.36)] in MenB IMD cases was observed in vaccine-eligible infants, irrespective of the infants' vaccination status or predicted meningococcal group B strain coverage.
In South Australia, more than 30 000 students aged 16 through 19 years (from 91% of high schools), received two doses of Bexsero with a one- to three-month interval. In an interrupted time-series analysis, a statistically significant reduction of 71% (95% CI: 15-90) in MenB IMD cases was observed in the two years of follow-up (July 2017-June 2019).
Immunogenicity
Serum bactericidal antibody responses to each of the vaccine antigens NadA, fHbp, NHBA and PorA P1.4 were evaluated using a set of four meningococcal group B reference strains. Bactericidal antibodies against these strains were measured by the Serum Bactericidal Assay using human serum as the source of complement (hSBA). Data are not available from all vaccine schedules using the reference strain for NHBA.
Most of the primary immunogenicity studies were conducted as randomized, controlled, multicenter, clinical trials. Immunogenicity was evaluated in infants, children, adolescents and adults.
Immunogenicity in infants and children
In infant studies, participants received three doses of Bexsero either at 2, 4 and 6 or 2, 3 and 4 months of age and a booster dose in their second year of life, as early as 12 months of age. Sera were obtained both before vaccination, one month after the third vaccination (see Table 2) and one month after booster vaccination (see Table 3). In an extension study the persistence of the immune response was assessed one year after the booster dose (see Table 3). The immunogenicity after two or three doses followed by a booster has been evaluated in infants 2 months to 5 months of age in another clinical study. The immunogenicity after two doses has been also documented in another study in infants 6 months to 8 months of age at enrolment (see Table 4).
Previously unvaccinated children also received two doses in the second year of life, with antibody persistence being measured at one year after the second dose (see Table 4).
Immunogenicity in infants 2 months to 5 months of age
Three-dose primary series followed by a booster
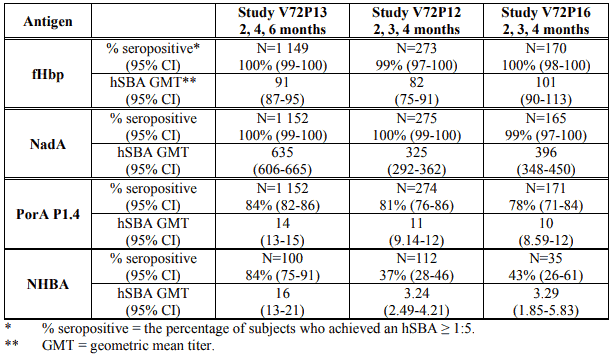
Immunogenicity results at one month after three doses of Bexsero administered at 2, 3, 4 and 2, 4, 6 months of age are summarised in Table 2. Bactericidal antibody responses one month after the third vaccination against meningococcal reference strains were high against the fHbp, NadA and PorA P1.4 antigens at both Bexsero vaccination schedules. The bactericidal responses against the NHBA antigen were also high in infants vaccinated at the 2, 4, 6-month schedule, but this antigen appeared to be less immunogenic at the 2, 3, 4-month schedule. The clinical consequences of the reduced immunogenicity of the NHBA antigen at this schedule are not known.
Table 2. Serum bactericidal antibody responses at 1 month following the third dose of Bexsero given at 2, 3, 4 or 2, 4, 6 months of age:
Data on bactericidal antibody persistence at 8 months after Bexsero vaccination at 2, 3 and 4 months of age, and at 6 months after Bexsero vaccination at 2, 4 and 6 months of age (pre-booster time point) and booster data after a fourth dose of Bexsero administered at 12 months of age are summarised in Table 3. Persistence of the immune response one year after the booster dose is also presented in Table 3.
Table 3. Serum bactericidal antibody responses following a booster at 12 months of age after a primary series administered at 2, 3 and 4 or 2, 4 and 6 months of age, and persistence of bactericidal antibody one year after the booster:
A decline in antibody titers to PorA P1.4 and fHbp antigens (reaching 9%-10% and 12%-20% of subjects with an hSBA ≥1:5, respectively) has been observed in an additional study in children 4 years of age who received a full priming and booster schedule as infants. In the same study the response to a further dose was indicative of immunological memory as 81%-95% of subjects reached an hSBA ≥1:5 to PorA P1.4 and 97%-100% to fHbp antigens following further vaccination. The clinical significance of this observation and the need for additional booster doses to maintain longer term protective immunity has not been established.
Two-dose primary series followed by a booster
The immunogenicity after two primary doses (at 3 and a half and 5 months of age) or three primary doses (at 2 and a half, 3 and a half and 5 months of age) of Bexsero followed by a booster dose in infants starting vaccination between 2 and 5 months of age has been evaluated in an additional phase 3 clinical study. The percentages of seropositive subjects (i.e. achieving an hSBA of at least 1:4) ranged from 44% to 100% one month after the second dose and from 55% to 100% one month after the third dose. At one month following a booster administered 6 months after the last dose, the percentages of seropositive subjects ranged from 87% to 100% for the two-dose schedule, and from 83% to 100% for the three-dose schedule.
Antibody persistence was evaluated in an extension study in children 3 to 4 years of age. Comparable percentages of subjects were seropositive at 2 to 3 years after being previously vaccinated with either two doses followed by a booster of Bexsero (ranging from 35% to 91%) or three doses followed by a booster (ranging from 36% to 84%). In the same study the response to an additional dose administered 2 to 3 years after the booster was indicative of immunological memory as shown by a robust antibody response against all Bexsero antigens, ranging from 81% to 100% and from 70% to 99%, respectively. These observations are consistent with adequate priming in infancy with both a two-dose and a threedose primary series followed by a booster of Bexsero.
Immunogenicity in infants 6 to 11 months and children 12 to 23 months of age
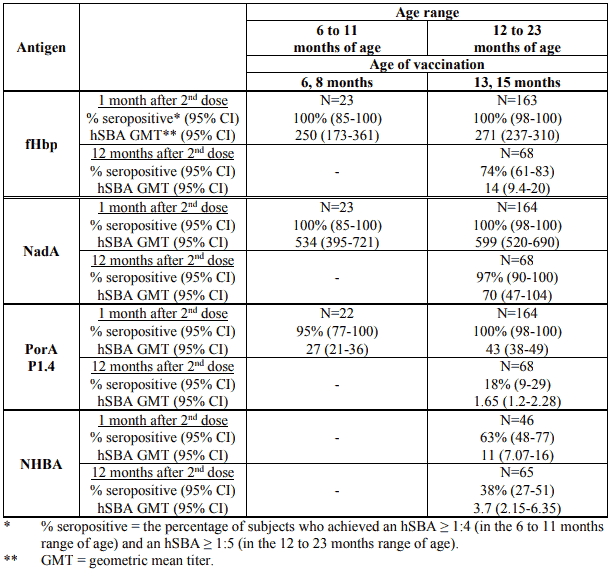
The immunogenicity after two doses administered two months apart in children 6 months to 23 months of age has been documented in two studies whose results are summarised in Table 4. Against each of the vaccine antigens, seroresponse rates and hSBA GMTs were high and similar after the two-dose series in infants 6-8 months of age and children 13-15 months of age. Data on antibody persistence one year after the two doses at 13 and 15 months of age are also summarised in Table 4.
Table 4. Serum bactericidal antibody responses following Bexsero vaccination at 6 and 8 months of age or 13 and 15 months of age and persistence of bactericidal antibody one year after the two doses at 13 and 15 months of age:
Immunogenicity in children 2 to 10 years of age
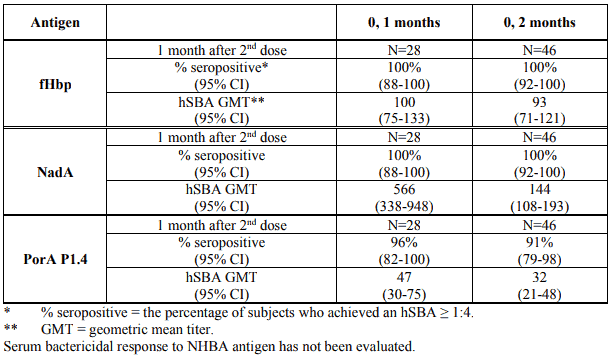
The immunogenicity after two doses of Bexsero administered either one or two months apart in children 2 to 10 years of age has been evaluated in an initial phase 3 clinical study and its extension. In the initial study, whose results are summarised in Table 5, participants received two doses of Bexsero two months apart. The seroresponse rates and hSBA GMTs were high after the two-dose schedule in children against each of the vaccine antigens (Table 5).
Table 5. Serum bactericidal antibody responses at 1 month following the second dose of Bexsero given to children 2-10 years of age following a 0, 2-month schedule:
In the extension study, in which two doses of Bexsero were administered one month apart in unvaccinated children, high percentages of subjects were seropositive one month after the second dose. An early immune response after the first dose was also evaluated. The percentages of seropositive subjects (i.e. achieving an hSBA of at least 1:4) across strains ranged from 46% to 95% at one month after the first dose and from 69% to 100% at one month after the second dose (Table 6).
Table 6. Serum bactericidal antibody responses at 1 month following the second dose of Bexsero given to children 2-10 years of age following a 0, 1-month schedule:
The same extension study also evaluated antibody persistence and the response to a booster dose in children who received the two-dose primary series at 2-5 or 6-10 years of age. After 24-36 months, the percentages of seropositive subjects (i.e. achieving an hSBA of at least 1:4) declined, ranging across strains from 21% to 74% in children 4-7 years of age and from 47% to 86% in children 8-12 years of age. The response to a booster dose administered 24-36 months after the primary series was indicative of immunological memory as the percentages of seropositive subjects ranged across strains from 93% to 100% in children 4-7 years of age and from 96% to 100% in children 8-12 years of age.
Immunogenicity in adolescents (from 11 years of age) and adults
Adolescents received two doses of Bexsero with one, two or six-month intervals between doses; these data are summarised in Tables 7 and 8.
In studies with adults, data were obtained after two doses of Bexsero with a one-month or two-month interval between doses (see Table 9).
The vaccination schedules of two doses administered with an interval of one or two months showed similar immune responses in both adults and adolescents. Similar responses were also observed for adolescents administered two doses of Bexsero with an interval of six months.
Table 7. Serum bactericidal antibody responses in adolescents one month after two doses of Bexsero administered according to different two-dose schedules and persistence of bactericidal antibody 18 to 23 months after the second dose:
In the study in adolescents, bactericidal responses following two doses of Bexsero were stratified by baseline hSBA less than 1:4 or equal to or greater than 1:4. Seroresponse rates and percentages of subjects with at least a 4-fold increase in hSBA titer from baseline to one month after the second dose of Bexsero are summarised in Table 8. Following Bexsero vaccination, a high percentage of subjects were seropositive and achieved 4-fold increases in hSBA titers independent of pre-vaccination status.
Table 8. Percentage of adolescents with seroresponse and at least 4-fold rise in bactericidal titers one month after two doses of Bexsero administered according to different two-dose schedules – stratified by pre-vaccination titers:
Antibody persistence data for the study in adolescents were obtained in an extension phase 3 study. At approximately 7.5 years after the two-dose primary series, the percentages of subjects with hSBA ≥1:4 declined, ranging across strains from 29% to 84%. The response to a booster dose administered 7.5 years after the primary series was indicative of immunological memory as the percentages of subjects reaching an hSBA ≥1:4 across strains ranged from 93% to 100%. The same study also evaluated antibody persistence data from an additional phase 3 initial study in adolescents. At approximately 4 years after the two-dose primary series, the percentages of subjects with hSBA ≥1:5 generally declined from a range across strains of 68% to 100% after the second dose to a range across strains of 9% to 84%. The response to a booster dose administered 4 years after the primary series was indicative of immunological memory as the percentages of subjects with hSBA ≥1:5 ranged across strains from 92% to 100%.
Table 9. Serum bactericidal antibody responses in adults after two doses of Bexsero administered according to different two-dose schedules:
Immunogenicity in special populations
Children and adolescents with complement deficiencies, asplenia, or splenic dysfunction
In a phase 3 clinical study, children and adolescents 2 through 17 years of age with complement deficiencies (40), with asplenia or splenic dysfunction (107), and age-matched healthy subjects (85) received two doses of Bexsero two months apart. At 1 month following the 2-dose vaccination course, the percentages of subjects with hSBA ≥1:5 in individuals with complement deficiencies and asplenia or splenic dysfunction were 87% and 97% for antigen fHbp, 95% and 100% for antigen NadA, 68% and 86% for antigen PorA P1.4, 73% and 94% for antigen NHBA, respectively, indicating an immune response in these immunocompromised subjects. The percentages of healthy subjects with hSBA ≥1:5 were 98% for antigen fHbp, 99% for antigen NadA, 83% for antigen PorA P1.4, and 99% for antigen NHBA.
5.2. Pharmacokinetic properties
Not applicable.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on repeated dose toxicity and reproductive and developmental toxicity studies.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.