BREYANZI Dispersion for infusion Ref.[49783] Active ingredients: Lisocabtagene maraleucel
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Bristol-Myers Squibb Pharma EEIG, Plaza 254, Blanchardstown Corporate Park 2, Dublin 15, D15 T867, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other antineoplastic agents
ATC code: L01XL08
Mechanism of action
Breyanzi is a CD19-directed genetically modified autologous cellular immunotherapy administered as a defined composition to reduce variability in CD8+ and CD4+ T-cell dose. The CAR is comprised of a murine FMC63 monoclonal antibody-derived single chain variable fragment (scFv), IgG4 hinge region, CD28 transmembrane domain, 4-1BB (CD137) costimulatory domain, and CD3 zeta activation domain. CD3 zeta signalling is critical for initiating T-cell activation and antitumour activity, while 4-1BB (CD137) signalling enhances the expansion and persistence of Breyanzi (see section 5.2).
CAR binding to CD19 expressed on the cell surface of tumour and normal B-cells induces activation and proliferation of CAR T-cells, release of pro-inflammatory cytokines, and cytotoxic killing of target cells.
Clinical efficacy and safety
TRANSFORM
The efficacy and safety of Breyanzi was compared to the standard of care (SOC) in a phase 3, randomised, open-label, parallel group, multicentre study, TRANSFORM (BCM-003), in adult patients with large B-cell non-Hodgkin lymphoma primary refractory to or relapsed within 12 months of initial therapy, who were candidates for HSCT. The SOC consisted of salvage immunochemotherapy followed by high dose chemotherapy (HDCT) and autologous HSCT. The study included patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), de novo or transformed indolent NHL, high grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with DLBCL histology (double/triple hit lymphoma [DHL/THL]), primary mediastinal large B-cell lymphoma (PMBCL), T cell/histiocyte rich large B cell lymphoma (THRBCL) or follicular lymphoma Grade 3B (FL3B), per WHO 2016 classification. The study included patients with ECOG performance status ≤ 1, and patients with secondary CNS lymphoma involvement could be enrolled in study BCM-003 if the individual patient benefit/risk was considered positive by the investigator.
Inclusion and exclusion criteria were chosen to ensure adequate organ function, and blood counts for HSCT. The study excluded patients with a creatinine clearance of less than 45 mL/min, alanine aminotransferase (ALT) >5 times the upper limit of normal (ULN) or left ventricular ejection fraction (LVEF) <40%, and absolute neutrophil count (ANC) <1.0 x 109 cells/L and platelets <50 x 109 cells/L in absence of bone marrow involvement.
Patients were randomised 1:1 to receive either Breyanzi or SOC. Randomisation was stratified by response to first-line therapy, and secondary age adjusted international prognostic index (sAAIPI) (0 to 1 versus 2 to 3). Patients randomised to Breyanzi were to receive lymphodepleting chemotherapy consisting of fludarabine 30 mg/m²/day and cyclophosphamide 300 mg/m²/day concurrently for 3 days followed by Breyanzi infusion 2 to 7 days after completion of lymphodepleting chemotherapy.
In the Breyanzi arm, bridging chemotherapy was permitted between apheresis and the start of lymphodepleting chemotherapy with 1 cycle of immunochemotherapy (i.e., rituximab, dexamethasone, cytarabine, and cisplatin [R-DHAP], rituximab, ifosfamide, carboplatin, and etoposide [R-ICE], or rituximab, gemcitabine, dexamethasone, and cisplatin [R-GDP]). All patients randomised to the SOC arm were to receive 3 cycles of salvage immunochemotherapy (i.e., R-DHAP, R-ICE, or R-GDP). Patients responding (complete response [CR] and partial response [PR]) after 3 cycles were to proceed to HDCT and autologous HSCT. Patients receiving SOC treatment were allowed to receive Breyanzi if they failed to achieve CR or PR after 3 cycles of salvage immunochemotherapy, or had disease progression at any time, or if the patient needed to start a new treatment due to efficacy concerns.
Of 92 patients randomised to Breyanzi, 58 (63%) received anticancer therapy for disease control (bridging therapy), 89 (97%) received Breyanzi and 1 (1%) patient received nonconforming product. Two patients did not receive Breyanzi. Of these 2 (2%) patients, 1 (1%) did not receive Breyanzi due to manufacturing failure, and 1 (1%) patient withdrew consent prior to treatment. The median dose of Breyanzi was 99.9 x 106 CAR-positive viable T-cells (range: 97-103 x 106 CAR-positive viable T-cells).
Of 92 patients randomised to SOC, 91 (99%) patients started treatment. One (1%) patient withdrew consent before starting treatment. Forty-three (47%) patients completed immunochemotherapy, HDCT and HSCT treatment. Fifty-eight (63%) of patients went on to receive Breyanzi after failing SOC treatment.
The efficacy analyses were based on the ITT analysis set (n=184), which was defined as all patients randomised to a treatment arm.
The median time from leukapheresis to product availability was 26 days (range: 19 to 84 days), and the median time from leukapheresis to infusion was 36 days (range: 25 to 91 days).
Table 4 summarises the baseline patient and disease characteristics in the TRANSFORM trial.
Table 4. Baseline demographic and disease-related characteristics for TRANSFORM (intention-to-treat [ITT] analysis set):
| Characteristic | Breyanzi (N=92) | SOC (N=92) |
|---|---|---|
| Median age, years (range) | 60.0 (20, 74) | 58.0 (26, 75) |
| ≥65 to <75 years, n (%) | 36 (39.1) | 23 (25.0) |
| ≥75 years, n (%) | 0 | 2 (2.2) |
| Sex, n (%) | ||
| Male | 44 (47.8) | 61 (66.3) |
| Female | 48 (52.2) | 31 (33.7) |
| ECOG Performance Status (at Screening) | ||
| ECOG 0, n (%) | 48 (52.2) | 57 (62.0) |
| ECOG 1, n (%) | 44 (47.8) | 35 (38) |
| Disease histology subtype, n (%) | ||
| DLBCL, NOS | 53 (57.6) | 50 (54.3) |
| DLBCL transformed from indolent lymphoma | 7 (7.6) | 8 (8.7) |
| High-grade B cell lymphoma | 22 (23.9) | 21 (22.8) |
| PMBCL | 8 (8.7) | 9 (9.8) |
| FL3B | 1 (1.1) | 0 |
| T cell rich/histiocyte rich large B-cell lymphoma | 1 (1.1) | 4 (4.3) |
| Chemorefractorya, n (%) | 26 (28.3) | 18 (19.6) |
| Refractoryb, n (%) | 67 (72.8) | 70 (76.1) |
| Relapsedc, n (%) | 25 (27.2) | 22 (23.9) |
| Confirmed CNS involvement, n (%) | 1 (1.1) | 3 (3.3) |
| Never achieved CR from prior therapies, n (%) | 62 (67.4) | 64 (69.6) |
a Chemorefractory is defined as experiencing stable disease (SD) or progressive disease (PD) to last chemo-containing regimen
b The status was refractory if a patient achieved SD, PD, PR or CR with relapse before 3 months.
c The status was relapsed if a patient achieved CR with relapse on or after lasting at least 3 months but no more than 12 months.
This study demonstrated statistically significant improvements in the primary endpoint of event free survival (EFS), and key secondary endpoints of complete response (CR) rate, and progression-free survival (PFS) for patients randomised to Breyanzi compared to SOC. Efficacy was based on EFS as determined by an independent review committee (IRC) using 2014 Lugano criteria. EFS was defined as the time from randomization to death from any cause, progressive disease, failure to achieve CR or PR by 9 weeks post-randomization (after 3 cycles of salvage immunochemotherapy and 5 weeks after Breyanzi infusion) or start of new antineoplastic therapy due to efficacy concerns, whichever occurs first. At a pre-specified interim analysis at 80% of the information fraction with a median on-study follow up time of 6.2 months (range 0.9 to 20 months), Breyanzi demonstrated a statistically significant improvement in EFS compared to the SOC arm (HR = 0.349 [95% CI: 0.229, 0.530], one-sided p-value <0.0001). The p-value was compared with 0.012 of the allocated alpha for the pre-specified interim analysis.
Breyanzi demonstrated an improvement compared to SOC in DLBCL (n=60, HR: 0.357 [95% CI: 0.204, 0.625]) and HGBCL (n=22, HR: 0.413 [95% CI: 0.189, 0.904]).
The results of the final analysis (shown in Table 5 and Figure 1), with a median on-study follow-up time of 33.86 months (range 0.9 to 53.0 months), were consistent with both the interim and primary analyses.
Table 5. TRANSFORM study: Response rate, event-free survival, progression-free survival and overall survival in patients with relapsed or refractory LBCL (ITT analysis set):
| Outcomea | Breyanzi arm (N=92) | SOC arm (N=92) |
|---|---|---|
| Event-free survival, (months) | ||
| Number of events n, (%) | 48 (52.2) | 73 (79.3) |
| Median [95% CI]b | 29.5 (9.5, NR) | 2.4 (2.2, 4.9) |
| Hazard ratio [95% CI]c | 0.375 [0.259, 0.542] | |
| Complete response rate | ||
| n (%) | 68 (73.9) | 40 (43.5) |
| Two sided [95% CI] | [63.7, 82.5] | [33.2, 54.2] |
| Progression free survival, (months) | ||
| Number of events n, (%) | 41 (44.6) | 54 (58.7) |
| Median [95% CI]b | NR (12.6, NR) | 6.2 (4.3, 8.6) |
| Hazard ratio [95% CI]c | 0.422 [0.279, 0.639] | |
| Overall survival (OS), (months) | ||
| Number of events n, (%) | 34 (37.0) | 42 (45.7) |
| Median [95% CI]b | NR (42.8, NR) | NR (18.2, NR) |
| Hazard ratio [95% CI]c | 0.757 [0.481, 1.191] | |
NR = not reached; CI = confidence interval.
a Per the Lugano criteria 2014, as assessed by an IRC.
b Kaplan-Meier estimate.
c Based on a stratified Cox proportional hazards model.
d Greenwood's formula.
Of the 92 patients in the Breyanzi arm, 80 (68 CR,12 PR) had a response with an overall response rate of 87%.
Figure 1. Kaplan-Meier plot of event-free survival based on IRC Assessment (ITT analysis set):
HR: Hazard ratio (stratified)
TRANSCEND-LBCL Cohort
The efficacy and safety of Breyanzi were evaluated in an open-label, multicentre, single-arm study, TRANSCEND (017001), in patients with relapsed or refractory (R/R) aggressive B-cell non-Hodgkin lymphoma (NHL). Eligible patients were ≥18 years with R/R DLBCL not otherwise specified (NOS), per WHO 2008 classification, including DLBCL arising from indolent lymphoma (transformed from follicular lymphoma, marginal zone lymphoma, chronic lymphocytic leukaemia/small lymphocytic leukaemia, Waldenström's macroglobulinaemia, or other), and high-grade B-cell lymphoma; primary mediastinal large B-cell lymphoma (PMBCL) and follicular lymphoma grade 3B (FL3B), who had received at least 2 lines of therapy or after autologous haematopoietic stem cell transplant. Patients with other subtypes of DLBCL have not been included in the study and benefit-risk have not been established. The study included patients with ECOG performance status ≤2, prior autologous and/or allogenic haematopoietic stem cell transplant (HSCT), and secondary CNS lymphoma involvement. Patients who received prior CD19-directed therapy were eligible, provided CD19-positivity was confirmed on a tumour biopsy at any time after CD19-directed therapy. The study excluded patients with a creatinine clearance of less than 30 mL/min, alanine aminotransferase >5 times the upper limit of normal or, left ventricular ejection fraction <40%.
There was no minimum requirement for blood counts; patients were eligible to enroll if they were assessed by the investigator to have adequate bone marrow function to receive lymphodepleting chemotherapy. See Table 6 for baseline demographic and disease-related characteristics.
Treatment consisted of lymphodepleting (LD) chemotherapy, fludarabine 30 mg/m²/day and cyclophosphamide 300 mg/m²/day for 3 days, followed by Breyanzi 2 to 7 days later.
Anticancer therapy for disease control (bridging therapy) was permitted between apheresis and lymphodepletion. Of the 229 patients treated with Breyanzi, 137 (60%) received anti-cancer therapy for disease control; the type and duration of bridging therapy was left to the discretion of the investigator.
The median time from leukapheresis to product availability was 24 days (range: 17 to 51 days). In addition, the median time from leukapheresis to infusion was 38.5 days (range: 27 to 156 days).
Of 298 patients who underwent leukapheresis for whom Breyanzi was manufactured in the dose range of 44-120 x 106 CAR-positive viable T-cells, 229 (77%) patients received Breyanzi and 69 (23%) patients did not. Of these 69 patients, there were 27 (39%) manufacturing failures including 2 patients who did not receive Breyanzi and 25 patients who received treatment with investigational product that did not meet release specifications. Forty-two (61%) other patients were not treated with Breyanzi, the most frequent reasons being death (n=29) or disease complications (n=6). Among the patients treated within the range of 44-120 x 106 CAR-positive viable T-cells, the median dose of Breyanzi was 87 x 106 CAR-positive viable T-cells.
The number of patients who were evaluable for efficacy was 216 (Efficacy set). Thirteen patients were not evaluable for efficacy, including 10 patients who did not have baseline positron emission tomography-positive (PET+) disease, or confirmation of PET+ disease after anticancer therapy for disease control by IRC, and 3 for other reasons.
Table 6 summarises the baseline patient and disease characteristics in the TRANSCEND study.
Table 6. Baseline demographic and disease-related characteristics for TRANSCEND:
| Characteristic | All leukapheresed (N=298) | Breyanzi-treated (N=229) |
|---|---|---|
| Median age, years (range) | 62.0 (18, 82) | 62.0 (18, 82) |
| ≥65 years, n (%) | 116 (38.9) | 89 (38.9) |
| ≥75 years, n (%) | 25 (8.4) | 19 (8.3) |
| Sex, n (%) | ||
| Male | 197 (66.1) | 153 (66.8) |
| Female | 101 (33.9) | 76 (33.2) |
| Prior HSCT, n (%) | 106 (35.6) | 87 (38.0) |
| Autologous HSCT | 100 (33.6) | 84 (36.7) |
| Allogeneic HSCT | 11 (3.7) | 8 (3.5) |
| ECOG performance status (at screening) | ||
| ECOG 0-1, n (%) | 290 (97.3) | 225 (98.3) |
| ECOG 2, n (%) | 8 (2.7) | 4 (1.7) |
| Disease histology subtype, n (%) | ||
| DLBCL, NOS | 142 (47.7) | 117 (51.1) |
| DLBCL transformed from indolent lymphoma | 87 (29.2) | 60 (26.2) |
| High-grade B cell lymphomaa | 48 (16.1) | 33 (14.4) |
| PMBCL | 15 (5.0) | 15 (6.6) |
| FL3B | 6 (2.0) | 4 (1.7) |
| Median number of prior therapies (range) | 3 (1-12) | 3 (1-8) |
| Chemorefractoryb, n (%) | 212 (71.1) | 160 (69.9) |
| Refractoryc, n (%) | 246 (82.6) | 186 (81.2) |
| Relapsedd, n (%) | 52 (17.4) | 43 (18.8) |
| Secondary CNS lymphoma at time of Breyanzi infusion, n (%) | 7 (2.3) | 6 (2.6) |
| Never achieved CR from prior therapies, n (%) | 141 (47.3) | 103 (45.0) |
a MYC and BCL2 and/or BCL6 rearrangements with DLBCL histology.
b Chemorefractory is defined as experiencing stable disease (SD) or progressive disease (PD) to last chemo-containing regimen or relapsed <12 months after autologous stem cell transplantation.
c The status was refractory if a patient achieved less than a complete response (CR) to last prior therapy.
d The status was relapsed if a patient achieved CR to last prior therapy.
Efficacy was assessed on the basis of the primary endpoint, overall response rate (ORR), and secondary endpoints which included CR rate, duration of response (DOR) as determined by an independent review committee (Table 7 and Figure 2). The median on-study follow-up time was 20.5 months (range 0.2 to 60.9 months).
Table 7. TRANSCEND study: Response rate, duration of response (IRC assessment):
| All leukapheresed (N=298) | Efficacy set (N=216) | |
|---|---|---|
| Overall response ratea, n (%) | 179 (60.1) | 157 (72.7) |
| [95% CI] | [54.3, 65.7] | [66.2, 78.5] |
| Complete response, n (%) | 128 (43.0) | 115 (53.2) |
| [95% CI] | [37.3, 48.8] | [46.4, 60.0] |
| Partial response, n (%) | 51 (17.1) | 42 (19.4) |
| [95% CI] | [13.0, 21.9] | [14.4, 25.4] |
| Duration of response (DOR)a,b (months) | n=179 | n=157 |
| Median | 16.8 | 20.5 |
| [95% CI]c | [8.0, NR] | [8.2, NR] |
| Range | 0.0, 34.3+ | 0.0, 34.3+ |
| DOR if best response is CRa,b (months) | n=128 | n=115 |
| Median | 26.1 | 26.1 |
| [95% CI]c | [23.1, NR] | [23.1, NR] |
| Range | 0.0, 34.3+ | 0.0, 34.3+ |
CI = confidence interval; CR = complete response; IRC = Independent Review Committee; KM = Kaplan-Meier; NR = not reached
a Per the Lugano 2014 criteria, as assessed by IRC.
b Deaths after initiation of anti-cancer treatment were considered as events.
c KM method was used to obtain 2-sided 95% CIs.
+ Ongoing.
The median time to response (CR or partial response [PR]) was 1.0 months (range: 0.7 to 8.9 months). The median time to CR was 1.0 months (range: 0.8 to 12.5 months). Response durations were longer in patients who achieved a CR, as compared to patients with a best response of PR.
Six patients with secondary CNS lymphoma were treated and evaluable for efficacy in the TRANSCEND study. Three of these six patients achieved a CR; 2 of 3 patients had durable remissions of 23 months that remained ongoing at study completion. The safety profile of these patients with secondary CNS lymphoma was consistent with that observed in the overall population.
In the Efficacy set, the ORR results within PMBCL and FL3B were 79% (11/14 patients) and 100% (4/4 patients) respectively. CR rates were 50% for PMBCL and 100% for FL3B. The safety profile was consistent across these subtypes.
In the Efficacy set, the ORR results within patients with DLBCL transformed (t) from prior indolent lymphoma of FL, marginal cell lymphoma (MZL), chronic lymphocytic leukaemia/small lymphocytic lymphoma; (CLL/SLL), and Waldenstrom macroglobulinemia (WM) were 86% (38/44 patients), 43% (3/7 patients), 50% (2/4 patients) and 50% (½ patients), respectively. CR rates were 61.4% for tFL, 29% for tMZL, 25% for tCLL/SLL (Richter's syndrome), and 0% for WM, respectively. The safety profile was consistent across these subtypes. Durable remissions (i.e. DOR ≥12 months) were observed in patients with tFL and tMZL, however, there is very limited experience for patients with tCLL/SLL (4 patients) and tWM (2 patients) in whom maximal DORs of 2 and 5.3 months, respectively were observed. The safety profile was consistent across these subtypes.
In clinical studies of Breyanzi, 89 (39%) of the 229 patients in TRANSCEND were 65 years of age or older, and 19 (8%) were 75 years of age or older. The safety or efficacy of Breyanzi observed between these patients and younger patients was similar.
Eleven patients received prior CD19-directed therapy and had efficacy and safety outcomes similar to the overall population. All patients had CD19 expression prior to Breyanzi infusion.
There is limited experience of the use of Breyanzi for patients with ECOG performance status of 2 prior to apheresis (4 patients), and prior allogeneic HSCT (8 patients).
Amongst 229 Breyanzi-treated patients, the majority of patients (n=209) received Breyanzi within the recommended CD4:CD8 ratio range of 0.8 to 1.2. There is limited experience of the use of Breyanzi outside this CD4:CD8 ratio range (n=19 above 1.2, n=1 below 0.8) which therefore limits the interpretation of the data in this subgroup.
Of the 115 patients who achieved CR, 82 (71%) had remission lasting at least 6 months and 74 (64%) had remission lasting at least 12 months.
Figure 2. Duration of response for responders per IRC assessment, TRANSCEND Efficacy set:
CR = complete response; PR = partial response.
Deaths after initiation of anti-cancer treatment were considered as events
Eleven patients with a history of hepatitis B or hepatitis C were treated with Breyanzi without hepatitis reactivation, while receiving antiviral suppressive therapy in accordance with clinical guidelines (see section 4.4).
TRANSCEND WORLD
TRANSCEND WORLD is an ongoing single-arm, multicentre, phase 2 study. Its Cohort 1 purpose is to provide clinical experience with Breyanzi in Europe for the treatment of adult patients 3L+ large B-cell lymphoma, defined as R/R DLBCL (DLBCL NOS [de novo], transformed FL), high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with DLBCL histology and FL3B per WHO 2016 classification. Patients previously treated with CD19-targeted therapy were excluded. See Table 8 below for baseline demographics and disease-related characteristics.
Table 8. Baseline demographic and disease-related characteristics for TRANSCEND WORLD (Cohort 1):
| Characteristic | All leukapheresed (N=45) | Breyanzi-treated (N=36) |
|---|---|---|
| Median age, years (range) | 64.0 (26, 73) | 61.5 (26.0, 72.0) |
| ≥65 years, n (%) | 19 (42.2) | 14 (38.9) |
| ≥75 years, n (%) | 0 | 0 |
| Sex, n (%) | ||
| Male | 30 (66.7) | 25 (69.4) |
| Female | 15 (33.3) | 11 (30.6) |
| Prior HSCT, n (%) | 14 (31.1) | 12 (33.3) |
| Autologous HSCT | 14 (31.1) | 12 (33.3) |
| Allogeneic HSCT | 0 | 0 |
| ECOG performance status (at screening) | ||
| ECOG 0, n (%) | 26 (57.8) | 19 (52.8) |
| ECOG 1, n (%) | 18 (40.0) | 16 (44.4) |
| ECOG 2, n (%) | 1 (2.2) | 1 (2.8) |
| Disease histology subtype, n (%) | ||
| DLBCL, NOS | 36 (80.0) | 31 (86.1) |
| High-grade B cell lymphomaa | 7 (15.6) | 4 (11.1) |
| PMBCL | 0 | 0 |
| FL3B | 2 (4.4) | 1 (2.8) |
| Chemorefractoryb, n (%) | 37 (82.2) | 29 (80.6) |
| Refractoryc, n (%) | 36 (80.0) | 28 (77.8) |
| Relapsedd, n (%) | 9 (20.0) | 8 (22.2) |
a MYC and BCL2 and/or BCL6 rearrangements with DLBCL histology.
b Chemorefractory is defined as experiencing stable disease (SD) or progressive disease (PD) to last chemo-containing regimen or relapsed < 12 months after autologous stem cell transplantation.
c The status was refractory if a patient achieved less than a complete response (CR) to last prior therapy.
d The status was relapsed if a patient achieved CR to last prior therapy.
At the time of the final analysis, 45 patients in Cohort 1 had been leukapheresed and 36 patients treated with Breyanzi, with a median follow-up time of 15.8 months. The median time from leukapheresis to product availability was 29 days (range: 24 to 38 days). In the Breyanzi-treated group, the ORR was 61.1% (95% CI: 43.5-76.9), and the CR rate was 33.3% (95% CI: 18.6-51.0). The disease burden and baseline demographics were indicative of advanced, aggressive disease characteristics. The safety profile of Breyanzi was consistent with the overall pooled safety population. See section 4.8 for adverse drug reactions associated with lisocabtagene maraleucel.
TRANSCEND-FL
The efficacy and safety of Breyanzi was evaluated in a Phase 2, open-label, multicentre, single-arm study (TRANSCEND-FL) in adult patients with relapsed or refractory FL grades 1, 2 and 3A after two or more lines of systemic therapy. The study enrolled patients with ECOG performance status of ≤1. The study excluded patients with a creatinine clearance of less than 30 mL/min, alanine aminotransferase >5 times the upper limit of normal or, left ventricular ejection fraction (LVEF) ≤40%. There was no prespecified threshold for blood counts; patients were eligible to enroll if they were assessed by the investigator to have adequate bone marrow function to receive lymphodepleting chemotherapy.
Treatment consisted of lymphodepleting (LD) chemotherapy, fludarabine 30 mg/m²/day and cyclophosphamide 300 mg/m²/day for 3 days, followed by Breyanzi 2 to 7 days later. The median dose of Breyanzi was 100 x 106 CAR-positive viable T-cells (range: 93.4-109.2 x 106 CAR-positive viable T-cells).
Anticancer therapy for disease control (bridging therapy) was permitted between apheresis and lymphodepletion. Of the 107 patients treated with Breyanzi, 44 (41%) received anticancer therapy, for disease control at the discretion of the investigator.
Of 114 patients who underwent leukapheresis, 107 (93.8%) patients received Breyanzi, and 4 (3.5%) patients received non-conforming product. Three (2.7%) patients did not receive Breyanzi for the following reasons: 1 (0.9%) patient due to an adverse event, 1 (0.9%) patient did not meet study criteria and 1 (0.9%) patient due to other reasons.
The number of patients who were evaluable for efficacy was 103 (efficacy set). Four patients were not evaluable for efficacy, as those patients did not have baseline PET-positive disease, or confirmation of PET-positive disease after anticancer therapy for disease control by IRC.
The median time from leukapheresis to product availability was 29 days (range: 20 to 55 days), and the median time from leukapheresis to product infusion was 50 days (range: 31 to 313 days).
Table 9. Baseline demographic and disease-related characteristics for TRANSCEND-FL:
| Characteristic | All leukapheresed (N=114) | Breyanzi-treated (N=107) |
|---|---|---|
| Median age, years (range) | 62.0 (23, 80) | 62.0 (23, 80) |
| ≥65 to <75 years, n (%) | 36 (31.6) | 32 (29.9) |
| ≥75 years, n (%) | 10 (8.8) | 10 (9.3) |
| Male gender, n (%) | 72 (63.2) | 66 (61.7) |
| Prior HSCT, n (%) Autologous HSCT | 34 (29.8) | 33 (30.8) |
| High FLIPI score (3-5), n (%) | 66 (57.9) | 61 (57.0) |
| Stage III-IV disease at screening, n (%) | 102 (89.4) | 95 (88.7) |
| ECOG performance status (at screening) | ||
| ECOG 0, n (%) | 68 (59.6) | 65 (60.7) |
| ECOG 1, n (%) | 46 (40.4) | 42 (39.3) |
| Double refractory, n (%) | 74 (64.9) | 69 (64.5) |
| Progression within 24 months of first line therapy with anti-CD20 and alkylator, n (%) | ||
| Yes | 63 (55.3) | 58 (54.2) |
| No | 50 (43.9) | 48 (44.9) |
| Not estimable | 1 (0.9) | 1 (0.9) |
| Median number of prior systemic treatments (range) | 3 (2,10) | 3 (2, 10) |
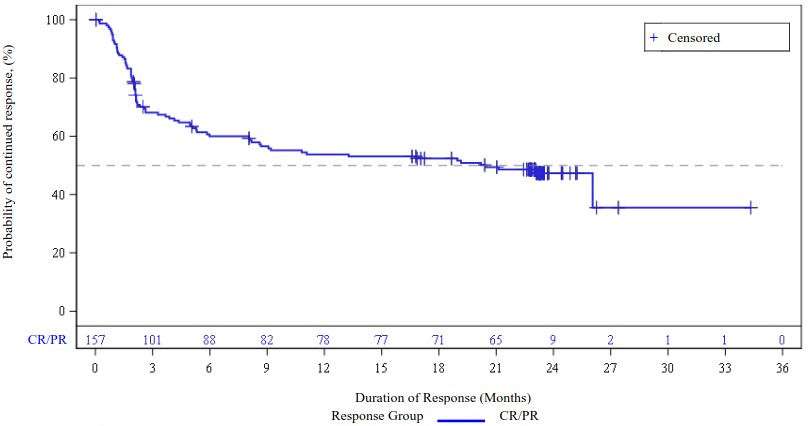
Efficacy was based on overall response rate (ORR), defined as the percentage of patients with a best overall response (BOR) of complete response (CR) or partial response (PR) after Breyanzi infusion as determined by an IRC (Table 10). The median on-study follow-up time was 30.0 months (range 0.3 to 39.6 months).
The median time to first response (CR or PR) and median time to first CR was 0.95 months (range: 0.6 to 3.3 months).
Table 10. TRANSCEND-FL study: Response rate, duration of response (IRC assessment):
| All leukapheresed (N=114) | Efficacy set (N=103) | |
|---|---|---|
| Overall response ratea, n (%) [95% CI]b | 106 (93.0) [86.6, 96.9] | 100 (97.1) [91.7, 99.4] |
| Complete response, n (%) [95% CI]b | 103 (90.4) [83.4, 95.1] | 97 (94.2) [87.8, 97.8] |
| Partial response, n (%) [95% CI]b | 3 (2.6) [0.5, 7.5] | 3 (2.9) [0.6, 8.3] |
| Duration of response (DOR) (months) | ||
| Median [95% CI]c | NR [30.85, N.R] | NR [30.85, NR] |
| Range | 1.9, 35.0+ | 1.9, 35.0+ |
| Rate of continued remissiond, % [95% CI] At 18 months | 76.1 (66.7, 83.2) | 75.7 (66.0, 83.0) |
CI = confidence interval; CR = complete response; NR = not reached;
+ indicates a censored value
a Per the Lugano criteria, as assessed by an IRC
b Two-sided 95% confidence interval based on exact Clopper-Pearson method.
c Median, Q1, Q3 are estimated from KM product-limit estimates
d Based on KM estimates of duration of response
Figure 3. Duration of response by IRC assessment, TRANSCEND-FL Efficacy set:
TRANSCEND-MCL Cohort
The efficacy and safety of Breyanzi were evaluated in an open-label, multicenter, single-arm trial (TRANSCEND-MCL Cohort) in adult patients with relapsed or refractory MCL who had received at least 2 prior lines of therapy including a Bruton's tyrosine kinase (BTK) inhibitor, an alkylating agent, and an anti-CD20 agent. The study included patients with ECOG performance status of ≤2, prior autologous and/or allogeneic HSCT, and secondary CNS lymphoma involvement. The study excluded patients with a creatinine clearance ≤30 mL/min, alanine aminotransferase >5 times the upper limit of normal or left ventricular ejection fraction (LVEF) <40%. There was no prespecified threshold for blood counts; patients were eligible to enroll if they were assessed by the investigator to have adequate bone marrow function to receive lymphodepleting chemotherapy.
Treatment consisted of LD chemotherapy, fludarabine 30 mg/m²/day and cyclophosphamide 300 mg/m²/day for 3 days, followed by Breyanzi 2 to 7 days later.
Anticancer therapy for disease control (bridging therapy) was permitted between apheresis and lymphodepletion. Of the 88 patients treated with Breyanzi, 58 (65.9%) received anticancer therapy for disease control, at the discretion of the investigator.
Of 104 patients who underwent leukapheresis, 88 (84.6%) patients received Breyanzi; the median dose of Breyanzi was 99.5 × 106 CAR-positive viable T-cells (range: 46-103 × 106 CAR positive viable T-cells). Four (3.8%) patients received non-conforming product. Twelve (11.5%) patients did not receive Breyanzi for the following reasons: 8 (7.6%) patients due to death, 1 (0.9%) patient no longer met eligibility criteria, and 3 (2.8%) patients due to other reasons.
Of the 88 patients who received Breyanzi, 81 patients were evaluable for efficacy and received at least 2 prior lines of systemic therapy including a BTK inhibitor, and were included in the efficacy set: five patients were not evaluable for efficacy as these patients did not have baseline PET-positive disease or confirmation of PET-positive disease after anticancer therapy for disease control by IRC, 1 patient did not receive at least 2 prior lines of systemic therapy and a BTK inhibitor and 1 patient did not receive a prior BTK inhibitor.
The median time from leukapheresis to product availability was 24.5 days (range: 17 to 80 days). In addition, the median time from leukapheresis to product infusion was 39 days (range: 28 to 489 days).
Table 11. Baseline demographic and disease-related characteristics for the TRANSCEND-MCL Cohort:
| Characteristic | All leukapheresed (N=104) | Breyanzi-treated (N=88) |
|---|---|---|
| Median age, years (range) | 68.0 (36, 86) | 68.5 (36, 86) |
| ≥65, n (%) | 71 (68.3) | 64 (72.7) |
| ≥75 years, n (%) | 22 (21.2) | 18 (20.5) |
| Sex, n (%) | ||
| Male | 81 (77.9) | 67 (76.1) |
| Female | 23 (22.1) | 21 (23.9) |
| Prior HSCT, n (%) | ||
| Autologous HSCT | 33 (31.7) | 26 (29.5) |
| Allogeneic HSCT | 8 (7.7) | 6 (6.8) |
| ECOG performance status (at screening) | ||
| ECOG 0, n (%) | 56 (53.8) | 48 (54.5) |
| ECOG 1, n (%) | 47 (45.2) | 40 (45.5) |
| ECOG 2, n (%) | 1 (1.0) | 0 |
| High risk features, n (%) | ||
| Ki67 proliferation fraction ≥30% | 82 (78.8) | 66 (75.0) |
| TP53 mutation | 25 (24.0) | 20 (22.7) |
| Blastoid morphology | 30 (28.8) | 27 (30.7) |
| Complex Karyotype | 30 (28.8) | 26 (29.5) |
| Secondary CNS lymphoma at time of Breyanzi infusion, n (%) | 7 (6.7) | 7 (8.0) |
| Median number of prior systemic treatments (range) | 3 (1, 11) | 3 (1, 11) |
| Refractory or relapsed to last prior therapy, n (%) | ||
| Refractorya | 70 (67.3) | 58 (65.9) |
| Relapsedb | 34 (32.7) | 30 (34.1) |
a The status was refractory if a patient achieved less than a complete response (CR) to last prior therapy.
b The status was relapsed if a patient achieved CR to last prior therapy.
Efficacy was based on overall response rate (ORR), defined as the percentage of patients with a best overall response (BOR) of complete response (CR) or partial response (PR) after Breyanzi infusion as determined by an IRC (Table 12). The median on-study follow-up time was 19.5 months (range 0.4 to 72 months).
Among the 81 patients included in the efficacy set, the median time to first response (CR or PR) was 0.95 month (range: 0.7 to 3.0 months) and the median time to first CR was 0.95 month (range: 0.7 to 4.9 months). Response durations were longer in patients who achieved a BOR of CR, as compared to patients with a BOR of PR.
Table 12. TRANSCEND-MCL Cohort: Response rate, duration of response (IRC assessment):
| All Leukapheresed (N=104) | Efficacy set (N=81) | |
|---|---|---|
| Overall response ratea, n (%) | 73 (70.2) | 67 (82.7) |
| [95% CI] | [60.4, 78.8] | [72.7, 90.2] |
| Complete response, n (%) | 64 (61.5) | 58 (71.6) |
| [95% CI]b | [51.5, 70.9] | [60.5, 81.1] |
| Partial response, n (%) | 9 (8.7) | 9 (11.1) |
| [95% CI]b | [4.0, 15.8] | [5.2, 20.0] |
| Number of responders Duration of response (DOR) (months) | ||
| Median [95% CI]c | 15.2 [7.0, 24.0] | 11.5 [6.2, 24.0] |
| Range | 0.0+, 24.0 | 0.0+, 24.0 |
| Rate of continued remissiond, % [95% CI] At 24 months | 44.8 (32.9; 55.9) | 41.2 (29.2, 52.9) |
| Median follow-up for DOR (months) | ||
| Median [95% CI] | 23.0 [22,8, 23.1] | 22.9 [22,8, 23.0] |
| Range | 0.0+, 24.0 | 0.0+, 24.0 |
CI = confidence interval; CR = complete response; NR = not reached;
+ indicates a censored value
a Per the Lugano 2014 criteria, as assessed by an IRC
b Two-sided 95% confidence interval based on exact Clopper-Pearson method.
c Median, Q1, Q3 are estimated from KM product-limit estimates
d Based on KM estimates of duration of response
Figure 4. Duration of response by IRC assessment, TRANSCEND-MCL Cohort efficacy set:
CR = complete response; PR = partial response.
PD/Death after initiation of anti-cancer treatment were considered as events
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Breyanzi in all subsets of the paediatric population in the treatment of mature B-cell neoplasms (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Following infusion, Breyanzi exhibited an initial expansion followed by a bi-exponential decline.
LBCL
In TRANSCEND-LBCL Cohort, in patients who received two or more prior lines of therapy for LBCL, the median time of maximal expansion in peripheral blood occurred 11 days after the first infusion. Breyanzi was present in peripheral blood for up to 2 years.
Among patients who received one prior line of therapy for LBCL (TRANSFORM), the median Cmax in responders (N=76) and non-responders (N=7) were 33,285 and 95,618 copies/μg, respectively. The median AUC0-28d in responders and non-responders were 268,887 and 733,406 day*copies/ μg, respectively.
In TRANSCEND, responders (N=150) had a 2.85-fold higher median Cmax than non-responders (N=45) (33,766.0 vs. 11,846.0 copies/μg). Responders had a 2.22-fold higher median AUC0-28d than non-responders (257,769.0 vs. 116,237.3 day*copies/μg).
In TRANSCEND, patients <65 years old (N=145) had a 2.93-fold and 2.35-fold higher median Cmax and AUC0-28d, respectively, compared to patients ≥65 years old (N=102, including 77 patients with age 65-74 years, 24 with age 75-84 years, and 1 with age ≥85 years). Sex and body weight did not show clear relationships to Cmax and AUC0-28d.
FL
In TRANSCEND-FL, in patients who received two or more prior lines of therapy for FL, the median time of maximal expansion in peripheral blood occurred 10 days after the first infusion. Breyanzi was present in peripheral blood for up to 3 years.
Among patients who received Breyanzi for FL (TRANSCEND-FL), the median Cmax in responders (N=100) and non-responders (N=2) were 31,336 and 15,568 copies/μg, respectively. The median AUC0-28d in responders (N=96) and non-responders (N=2) were 245,730 and 161,935 day*copies/μg, respectively.
MCL
In the TRANSCEND-MCL Cohort, in patients who received two or more prior lines of therapy for MCL, the median time of maximal expansion in peripheral blood occurred 10 days after the first infusion. Breyanzi was present in peripheral blood for up to 2 years.
Among patients who received Breyanzi for MCL (TRANSCEND-MCL Cohort), the median Cmax in responders (N=67) and non-responders (N=8) were 31,631 and 12,444 copies/μg, respectively. The median AUC0-28d in responders (N=67) and non-responders (N=8) were 309,578 and 142,462 day*copies/μg, respectively.
5.3. Preclinical safety data
Genotoxicity assays and carcinogenicity studies were not conducted.
In vitro expansion studies from healthy donors and patients showed no evidence for transformation and/or immortalization and no preferential integration near genes of concern in Breyanzi T cells.
Given the nature of the product, non-clinical studies on fertility, reproduction and development were not conducted.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.