BRINEURA Solution for infusion Ref.[7582] Active ingredients: Cerliponase alfa
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: BioMarin International Limited, Shanbally, Ringaskiddy, County Cork, Ireland
Therapeutic indications
Brineura is indicated for the treatment of neuronal ceroid lipofuscinosis type 2 (CLN2) disease, also known as tripeptidyl peptidase 1 (TPP1) deficiency.
Posology and method of administration
Brineura must only be administered by a trained healthcare professional knowledgeable in intracerebroventricular administration in a healthcare setting.
Posology
The recommended dose is 300 mg cerliponase alfa administered once every other week by intracerebroventricular infusion.
In patients less than 2 years of age, lower doses are recommended, see paediatric population section.
Pre-treatment of patients with antihistamines with or without antipyretics is recommended 30 to 60 minutes prior to the start of infusion.
Continuation of long-term treatment should be subject to regular clinical evaluation whether the benefits are considered to outweigh the potential risks to individual patients.
Dose adjustments
Consideration of dose adjustments may be necessary for patients who may not tolerate the infusion. The dose may be reduced by 50% and/or the infusion rate decreased to a slower rate.
If the infusion is interrupted due to a hypersensitivity reaction, it should be restarted at approximately one- half the initial infusion rate at which the hypersensitivity reaction occurred.
The infusion should be interrupted and/or the rate slowed in patients who in the judgement of the treating physician have a possible increase in intracranial pressure during the infusion as suggested by symptoms such as headache, nausea, vomiting, or decreased mental state. These precautions are of particular importance in patients below 3 years of age.
Paediatric population
The safety and efficacy of Brineura in children less than 3 years of age has not yet been established. Limited data are available for children aged 2 years and no clinical data is available in children below 2 years of age (see section 5.1). The posology proposed in children below 2 years has been estimated based on brain mass.
Treatment of Brineura was initiated in children 2 to 8 years of age in clinical studies. There is limited data in patients older than 8 years of age. Treatment should be based on the benefits and risks to the individual patient as assessed by the physician.
The posology selected for patients is based on age at time of treatment and should be adjusted accordingly (see Table 1). In patients less than 3 years of age the recommended dose is in accordance with the posology used in the ongoing clinical study 190-203, see section 5.1.
Table 1. Dose and volume of Brineura:
| Age groups | Total dose administered every other week (mg) | Volume of Brineura solution (ml) |
|---|---|---|
| Birth to <6 months | 100 | 3.3 |
| 6 months to <1 year | 150 | 5 |
| 1 year to <2 years | 200 (first 4 doses) | 6.7 (first 4 doses) |
| 300 (subsequent doses) | 10 (subsequent doses) | |
| 2 years and older | 300 | 10 |
Method of administration
Intracerebroventricular use.
Precautions to be taken before handling or administering the medicinal product
Aseptic technique must be strictly observed during preparation and administration.
Brineura and the flushing solution must only be administered by the intracerebroventricular route. Each vial of Brineura and flushing solution are intended for single use only.
Brineura is administered to the cerebrospinal fluid (CSF) by infusion via a surgically implanted reservoir and catheter (intracerebroventricular access device). The intracerebroventricular access device must be implanted prior to the first infusion. The implanted intracerebroventricular access device should be appropriate for accessing the cerebral ventricles for therapeutic administration.
Following Brineura infusion, a calculated amount of flushing solution must be used to flush the infusion components including the intracerebroventricular access device in order to fully administer Brineura and to maintain patency of the intracerebroventricular access device (see section 6.6). Brineura and flushing solution vials should be thawed prior to administration. The infusion rate for Brineura and the flushing solution is 2.5 ml/hour. The complete infusion time, including Brineura and the required flushing solution, is approximately 2 to 4.5 hours, depending on the dose and volume administered.
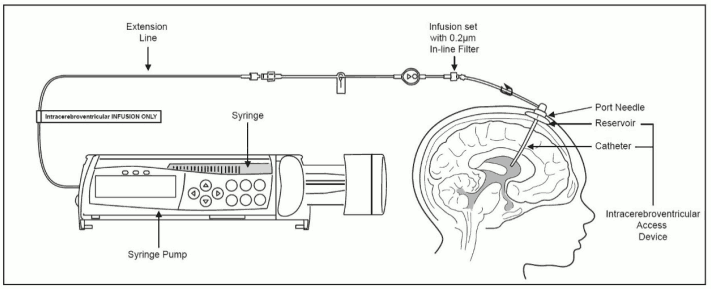
Intracerebroventricular Infusion of Brineura
Administer Brineura before the flushing solution.
- Label the infusion line for “Intracerebroventricular infusion only”.
- Attach the syringe containing Brineura to the extension line, if used, otherwise connect the syringe to the infusion set. The infusion set must be equipped with a 0.2 μm inline filter. See Figure 1.
- Prime the infusion components with Brineura.
- Inspect the scalp for signs of intracerebroventricular access device leakage or failure and for potential infections. Do not administer Brineura if there are signs and symptoms of acute intracerebroventricular access device leakage, device failure, or device-related infection (see section 4.3 and 4.4).
- Prepare the scalp for intracerebroventricular infusion using aseptic technique per institution standard of care.
- Insert the port needle into the intracerebroventricular access device.
- Connect a separate empty sterile syringe (no larger than 3 ml) to the port needle. Withdraw 0.5 ml to 1 ml of CSF to check patency of the intracerebroventricular access device.
- Do not return CSF to the intracerebroventricular access device. CSF samples should routinely be sent for infection monitoring (see section 4.4).
- Attach the infusion set to the port needle (see Figure 1).
- Secure the components per institution standard of care.
- Place the syringe containing Brineura into the syringe pump and program the pump to deliver at an infusion rate of 2.5 ml per hour.
- Program the pump alarms to sound at the most sensitive settings for pressure, rate, and volume limits. See the syringe pump manufacturer’s operating manual for details.
- Do not deliver as a bolus or manually.
- Initiate infusion of Brineura at a rate of 2.5 ml per hour.
- Periodically inspect the infusion system during the infusion for signs of leakage or delivery failure.
- Verify that the “Brineura” syringe in the syringe pump is empty after the infusion is complete. Detach and remove the empty syringe from the pump and disconnect from the tubing. Discard the empty syringe in accordance with local requirements.
Figure 1. Infusion System Set Up:
Intracerebroventricular infusion of the flushing solution
Administer the flushing solution provided after the Brineura infusion is complete.
- Attach the syringe containing the calculated volume of flushing solution to the infusion components (see section 6.6).
- Place the syringe containing the flushing solution into the syringe pump and program the pump to deliver an infusion rate of 2.5 ml per hour.
- Program the pump alarms to sound at the most sensitive settings for pressure, rate, and volume limits. See the syringe pump manufacturer’s operating manual for details.
- Do not deliver as a bolus or manually.
- Initiate infusion of the flushing solution at a rate of 2.5 ml per hour.
- Periodically inspect the infusion components during the infusion for signs of leakage or delivery failure.
- Verify that the “flushing solution” syringe in the syringe pump is empty after the infusion is complete. Detach and remove the empty syringe from the pump and disconnect from the infusion line.
- Remove the port needle. Apply gentle pressure and bandage the infusion site per institution standard of care.
- Dispose of the infusion components, needles, unused solutions and other waste materials in accordance with local requirements.
For instructions on preparation of Brineura and flushing solution before administration, see section 6.6.
Overdose
No information is available.
Shelf life
Shelf life: 2 years.
Thawed Brineura and flushing solution should be used immediately. Product should only be withdrawn from the unopened vials immediately prior to use. If immediate use is not possible, unopened vials of Brineura or flushing solution should be stored at 2-8°C and used within 24 hours.
Chemical and physical in-use stability has been demonstrated for up to 12 hours at room temperature (19-25°C). From a microbiological point of view, open vials or medicinal product held in syringes should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
Special precautions for storage
Store upright in a freezer (-25°C to -15°C).
Transport and distribute frozen (-85°C to -15°C).
Store in the original package in order to protect from light.
Nature and contents of container
Vial (type I glass) with a stopper (butyl rubber), a flip-off cap (polypropylene) and crimp seal (aluminium). Brineura has a green flip-off cap and flushing solution has a yellow flip-off cap.
Pack size of three vials: two 10 ml vials, each containing 150 mg of cerliponase alfa in 5 ml of solution; and one 10 ml vial, containing 5 ml flushing solution.
Special precautions for disposal and other handling
Brineura should be administered with infusion components shown to be chemically and physically compatible with administration of Brineura and flushing solution. CE marked intracerebroventricular access devices, and disposable components listed below or equivalent should be used to deliver Brineura.
Intracerebroventricular access devices shown to be compatible with Brineura and flushing solution and used in Brineura clinical studies include CodmanHOLTER RICKHAM and HOLTER SALMON-RICKHAM Reservoirs, Codman Ventricular Catheter, and Medtronic CSF-Ventricular Reservoir (with catheter).
Brineura is compatible with disposable infusion components made of PVC, PVC (non-DEHP) polyethylene, polyethersulfone (PES), polypropylene (PP), and PTFE. The following CE marked disposable infusion components were used in Brineura clinical trials:
- Syringe: Braun and BD Luer-Lok
- Extension Set: Fresenius Injectomat line, Alaris CC Extension set, Vygon Lectro-Cath Extension tube
- Extension Set with 0.2 micron filter: Impromediform GmbH
- Port needle: Deltec GRIPPER Needles
Preparation for administration of Brineura and flushing solution
The following components (not supplied) are required for proper administration of Brineura and flushing solution (see Figure 1 in section 4.2). All infusion components must be sterile. Brineura and flushing solution are supplied and stored frozen (see section 6.4).
- A programmable syringe pump with appropriate delivery range, delivery rate accuracy, and alarms for incorrect delivery or occlusion. The pump must be programmable to deliver the medicinal product at a constant rate of 2.5 ml/hr.
- Two single-use syringes compatible with the pump equipment. A syringe volume of 10 to 20 ml is recommended.
- Two single-use hypodermic syringe needles, (21 G, 25.4 mm).
- One single-use infusion set. An extension line may be added if needed. A length of 150 to 206 cm (not to exceed 400 cm) and an inner diameter of 0.1 cm is recommended.
- A 0.2 μm inline filter is required. The inline filter may be integral to the infusion set. The inline filter should be placed as close as practically possible to the port needle.
- A non-coring port needle with a gauge of 22 or smaller and a suggested length of 16 mm. Refer to the intracerebroventricular access device manufacturer’s recommendation for the port needle.
- One empty sterile single-use syringe (for collection of CSF to check patency).
Thaw Brineura and flushing solution
Thaw Brineura vials and flushing solution vial at room temperature for approximately 60 minutes. Do not thaw or warm vials any other way. Do not shake vials. Condensation will occur during thawing period. Thawing the vials outside the carton is recommended.
Brineura and flushing solution must be completely thawed and used immediately (see section 6.3).
Do not re-freeze vials or freeze syringes containing Brineura or flushing solution.
Inspect thawed Brineura and flushing solution vials
Inspect the vials to ensure they are fully thawed. Brineura solution should be clear to slightly opalescent and colourless to pale yellow. Brineura vials may occasionally contain thin translucent fibres or opaque particles. These naturally occurring particles are cerliponase alfa. These particles are removed via the 0.2 μm inline filter without having a detectable effect on the purity or strength of Brineura.
The flushing solution may contain particles that dissolve when the vial is fully thawed. The flushing solution should be clear and colourless.
Do not use if the solutions are discoloured or if there is other foreign particulate matter in the solutions.
Withdraw Brineura
Label one unused sterile syringe “Brineura” and attach a syringe needle. Remove the green flip-off caps from both Brineura vials. Using aseptic technique, withdraw the volume of Brineura solution per required dose (see Table 1 in section 4.2) into the sterile syringe labelled “Brineura”. Do not dilute Brineura. Do not mix Brineura with any other medicinal product. Discard the needle and empty vials per local requirements.
Withdraw flushing solution
Determine the volume of flushing solution needed to ensure complete delivery of Brineura to the cerebral ventricles. Calculate the flush volume by adding the priming volume of all infusion components, including the intracerebroventricular access device.
Label one unused sterile syringe “flushing solution” and attach a syringe needle. Remove the yellow flip-off cap from the flushing solution vial. Using aseptic technique, withdraw the appropriate amount of flushing solution from the vial into the new sterile syringe labelled “flushing solution”. Discard the needle and the vial with the remaining solution per local requirements.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.