CEJEMLY Concentrate for solution for infusion Ref.[112953] Active ingredients: Sugemalimab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: SFL Pharmaceuticals Deutschland GmbH, Marie-Curie-Strasse 8, 79539 Loerrach, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, monoclonal antibodies and antibody drug conjugates, PD-1/PD-L1 (Programmed cell death protein 1/death ligand 1) inhibitors
ATC code:L01FF11
Mechanism of action
Sugemalimab is a fully human immunoglobulin G4 monoclonal antibody. It specifically binds to programmed cell death ligand 1 (PD-L1), thus blocking its ligation with PD-1. PD-L1, when expressed on tumour cells and tumour-infiltrating immune cells, can contribute to the inhibition of an anti-tumour immune response. Binding of PD-L1 to the PD-1 and CD80 (B7.1) receptors found on T-cells and antigen presenting cells suppresses cytotoxic T-cell activity, T-cell proliferation, and cytokine production. Blockade of PD-L1/PD-1 and PD-L1/CD80 interactions releases the inhibition of immune responses without inducing antibody dependent cell-mediated cytotoxicity (ADCC).
Clinical efficacy and safety
The efficacy and safety of sugemalimab in combination with platinum-based chemotherapy for the treatment of adults aged ≥18 years with histologically or cytologically confirmed metastatic (stage IV) squamous or non-squamous NSCLC without sensitising EGFR mutations, ALK fusions, ROS1, or RET translocations was studied in a randomised, double-blind, placebo-controlled phase 3 study (GEMSTONE-302). Apart from testing for EGFR mutational status in participants with non-squamous NSCLC, testing for genomic tumour aberrations/oncogenic drivers was not mandatory for enrolment. Participants had to provide formalin-fixed tumour tissue samples for PD-L1 assay. The PD-L1 expression was evaluated at a central laboratory by immunohistochemistry using the Ventana PD-L1 (SP263) assay on a BenchMark autostainer assay (Roche Tissue Diagnostics, Oro Valley, AZ, USA) according to the manufacturer's instructions. Participants were excluded if they had history of autoimmune disease, administration of systemic immunosuppressive medicinal product within 2 weeks prior to randomisation and active or untreated CNS metastases.
The primary endpoint of this study was progression-free survival (PFS) assessed by investigator according to RECIST v1.1. The secondary endpoints included overall survival (OS), PFS in participants with PD-L1 expression ≥ 1% (as assessed by investigators according to RECIST v1.1), objective response rate (ORR) assessed by investigators according to RECIST v1.1, and duration of response (DoR). The type I error was controlled using sequential testing method in the order of PFS, OS, PFS in participants with PD-L1 expression ≥ 1%, and ORR.
A total of 479 participants were randomly (2:1) assigned to receive:
- for squamous NSCLC, sugemalimab 1200 mg with carboplatin AUC = 5 mg/mL/min and paclitaxel 175 mg/m² intravenously administered every 3 weeks for up to 4 cycles, followed by sugemalimab 1200 mg every 3 weeks
- for non-squamous NSCLC, sugemalimab 1200 mg with carboplatin AUC = 5 mg/mL/min and pemetrexed 500 mg/m² intravenously administered every 3 weeks for up to 4 cycles followed by sugemalimab 1200 mg and pemetrexed 500 mg/m² every 3 weeks
or
- placebo plus the same platinum-based chemotherapy regimens for squamous or non-squamous NSCLC as the group receiving sugemalimab for up to 4 cycles; then followed by placebo for squamous NSCLC, or placebo plus pemetrexed for non-squamous NSCLC.
The maximum duration of treatment with sugemalimab or placebo was 35 cycles (approximately 2 years) or until progressive disease, unacceptable toxicity, withdrawal of informed consent, death, or other reasons stipulated in the protocol.
Participants receiving placebo plus chemotherapy who experienced radiographic disease progression confirmed by investigator were able to cross-over to receive sugemalimab monotherapy.
During the first year of the treatment period, imaging assessments were performed at the 6th week and the 12th week after the first dose, and every 9 weeks thereafter; after 1 year: imaging assessments were performed every 12 weeks until disease progression, loss to follow-up, death, or end of study, whichever occurred first.
All participants were Asian and had Stage IV NSCLC; the median age was 63.0 years; 80.0% were males; 73.3% were former or current smokers; 38.8% were ≥65 years; 40.1% had squamous NSCLC; 59.9% had non-squamous NSCLC; 60.8% had PD-L1 expression ≥1% of the tumour; 11.9% had liver metastases at baseline; 14.0% had brain metastases at baseline; 82.5% had an ECOG performance status of 1.
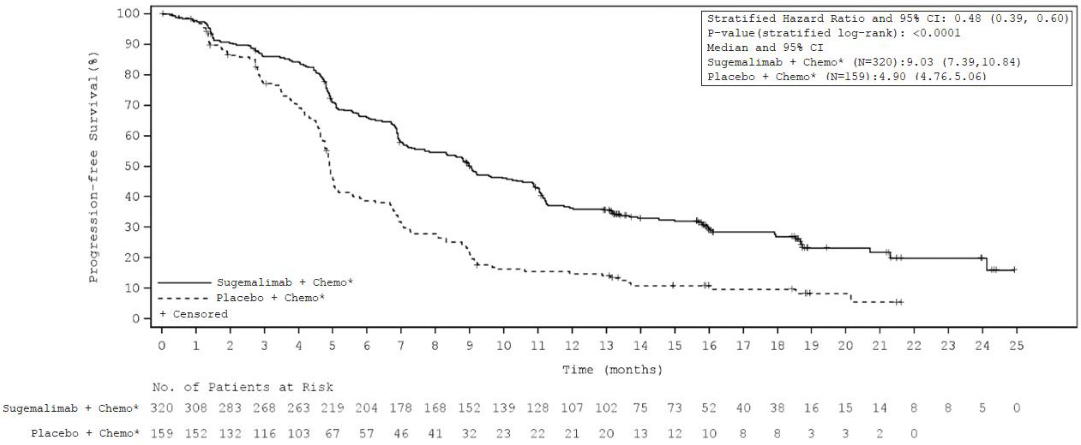
The median treatment duration was 10 cycles (range 1 to 49) with a median duration of 7.15 months for sugemalimab versus 6 cycles (range 1 to 44) with a median duration of 4.6 months for placebo. The efficacy results of the GEMSTONE-302 study are summarised in Table 3, Figure 1, and Figure 2.
Table 3. Efficacy results of the GEMSTONE-302 study:
| Efficacy endpoints | Sugemalimab in combination with platinum- based chemotherapy (n=320) | Placebo in combination with chemotherapy (n=159) |
|---|---|---|
| Progression free survival (PFS)* | ||
| Number (%) participants with event | 223 (69.7%) | 135 (84.9%) |
| Median in months (95% CI) | 9.0 (7.4, 10.8) | 4.9 (4.8, 5.1) |
| Hazard ratio (95% CI)† | 0.48 (0.39, 0.60) | |
| p-value† | <0.0001 | |
| Overall survival (OS) | ||
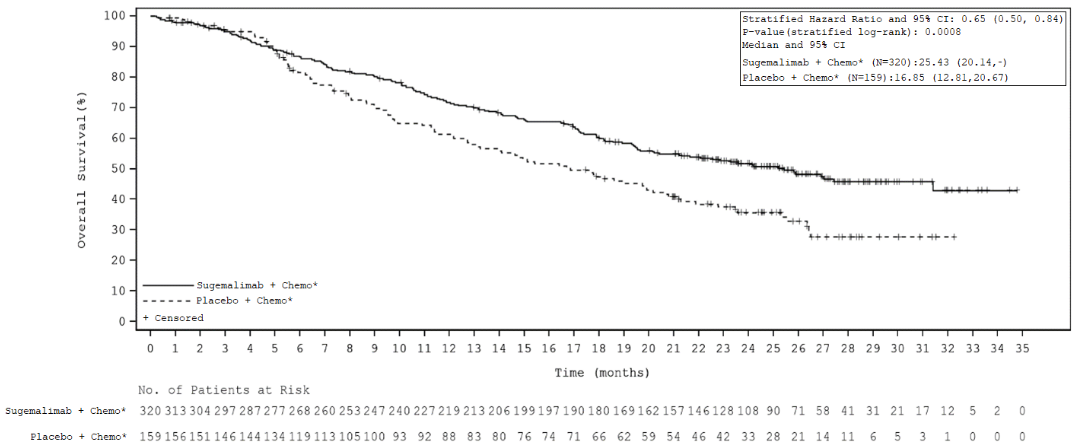
| Number (%) participants with event | 156 (48.8%) | 97 (61.0%) |
| Median in months (95% CI)¶ | 25.4 (20.1, NR) | 16.9 (12.8, 20.7) |
| Hazard ratio (95% CI)† | 0.65 (0.50, 0.84) | |
| p-value† | 0.0008 | |
| Objective response rate* | ||
| ORR n (%) (95% CI) | 203 (63.4%) (57.9, 68.7) | 64 (40.3%) (32.6, 48.3) |
| p-value§ | <0.0001 | |
CI = Confidence interval, ORR= Objective response rate
* Investigator assessed
† Hazard ratio (HR) is based on the stratified Cox model. P-value is based on the stratified log-rank test. The 3 stratification factors are ECOG performance status, PD-L1, and histology type from randomisation. See below for further explanation of histology type.
§ P value based on Cochran-Mantel-Haenszel test stratified by ECOG performance status, histology type and PD-L1 from randomisation.
Figure 1. Kaplan-Meier curve for investigator assessed progression-free survival – ITT population – study GEMSTONE-302:
Figure 2. Kaplan-Meier curve of overall survival – ITT population – study GEMSTONE-302:
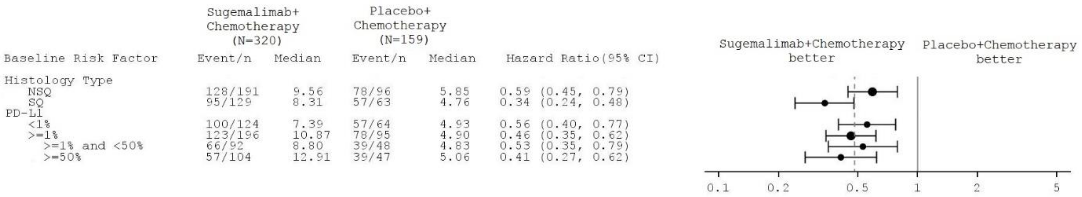
Figure 3. Forest-plot of PFS – study GEMSTONE-302:
Note: the subgroup analyses were not controlled for type 1 error.
Subgroup analysis showed improvements in PFS with sugemalimab, regardless of histological subtype and PD-L1, expression consistent with the overall intent-to-treat (ITT) population.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with sugemalimab in the paediatric population in the treatment of lung cancer (see section 4.2 for information on paediatric use).
Immunogenicity
In the phase 3 NSCLC study, the prevalence of anti-drug antibodies (ADA) was 17% (53 patients), with 9% (28 patients) as treatment-emergent ADA. No evidence was observed that ADA had an impact on pharmacokinetics, efficacy or safety, however data are still limited.
5.2. Pharmacokinetic properties
The PK of sugemalimab was characterised using population PK (PopPK) analysis with concentration data collected from 1002 participants who received sugemalimab doses in the range of 3 to 40 mg/kg and fixed dose of 1200 mg intravenous every 3 weeks.
Absorption
Sugemalimab is administered by intravenous infusion and therefore is immediately and completely bioavailable.
Following single- and multiple-doses escalation study of sugemalimab (n=29), sugemalimab exposures (AUC and Cmax) increased in an approximately dose proportional manner within the dosing range of 3 mg/kg to 40 mg/kg, including a fixed dose of 1200 mg intravenous every 3 weeks.
Following multiple intravenous infusions of 1200 mg every 3 weeks (n=16), there was approximately 2-fold accumulation of sugemalimab exposures (i.e., Racc,Cmax and Racc,AUC were 1.74 and 2.00, respectively).
Distribution
Consistent with a limited extravascular distribution of monoclonal antibodies, the volume of distribution of sugemalimab at steady-state (Vss) from popPK analysis was small, with a geometric mean (CV%) Vss of 5.56 L (21%) in patients with Stage IV NSCLC from Study GEMSTONE-302.
Biotransformation
As an antibody, sugemalimab is catabolised through non-specific pathways; metabolism does not contribute to its clearance.
Elimination
In the PopPK analysis, geometric mean (CV%) of total clearance (CL) after a single dose was estimated to be 0.235 L/day (24.2%) on NSCLC patients from Study GEMSTONE-302. At steady state, the elimination is slightly lower than after a single dose due to target-mediated drug disposition. Geometric mean (CV%) of the elimination half-life (t1/2) estimated from the PopPK model was approximately 17.9 days (25.6%) at the end of cycle 1 in NSCLC patients from Study GEMSTONE-302.
Special populations
Age, sex, body weight, tumour type, and anti-drug antibodies status
PopPK analysis showed non-statistically significant covariate effects of age (18-78 years) on sugemalimab exposure. The effect of other covariates (albumin, sex, anti-drug antibodies, and tumour type) on the systemic exposure of sugemalimab were not considered clinically meaningful. Based on the results from modelling and simulations, increasing the dosage to 1500 mg Q3W for patients with body weight more than 115 kg is anticipated to achieve comparable exposures to the patients in the pivotal study GEMSTONE-302 that were dosed with 1200 mg Q3W.
Race
The effect of race in participants with advanced solid tumours (including NSCLC) receiving sugemalimab was evaluated by PopPK analysis and no impact of race was identified on the PK of sugemalimab. More specifically, there was no observed PK difference in sugemalimab between Asian and non-Asian participants.
Hepatic impairment
The effect of mild hepatic impairment on sugemalimab PK was evaluated using PopPK analyses. Covariate analysis indicated no statistically significant effect of markers of liver function (AST and ALT) on sugemalimab exposure.
Renal impairment
The effect of renal impairment on the clearance of sugemalimab was evaluated using PopPK analyses in participants with mild or moderate renal impairment compared to participants with normal renal function. There was no impact of renal function on PK of sugemalimab.
5.3. Preclinical safety data
No carcinogenicity or reproductive toxicity studies have been conducted with sugemalimab.
Based on literature assessment, the PD-L1/PD-1 signalling pathway plays a role in pregnancy by maintaining maternal immune tolerance to the foetus. In a mouse model of pregnancy, blocking PD-L1 signalling can destroy the immune tolerance to the foetus and increase foetal miscarriages. No foetal malformations associated with blocking PD-L1/PD-1 signalling pathway have been reported in the literature, but immune-related diseases have been observed in PD-1 and PD-L1 gene knockout mice. Based on its mechanism of action, foetal exposure to sugemalimab may increase the risk of developing immune-related disorders or altering normal immune responses.
In 4- and 26-week repeat-dose toxicity studies in cynomolgus monkeys, exposure to sugemalimab administered IV once weekly, reveals no special hazard except two ophthalmic observations in high dose females: 1 incidence of retinal depigmentation and 1 case of medium-sized focal cornea opacity at 200 mg/kg, which corresponds to approximately 16-fold and 18-fold the clinical AUC at human recommended clinical dose.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.