CIBINQO Film-coated tablet Ref.[28174] Active ingredients: Abrocitinib
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other dermatological preparations, agents for dermatitis, excluding corticosteroids
ATC code: D11AH08
Mechanism of action
Abrocitinib is a Janus kinase (JAK)1 inhibitor. JAKs are intracellular enzymes which transmit signals arising from cytokine or growth factor-receptor interactions on the cellular membrane to influence cellular processes of haematopoiesis and immune cell function. JAKs phosphorylate and activate Signal Transducers and Activators of Transcription (STATs) which modulate intracellular activity including gene expression. Inhibition of JAK1 modulates the signalling pathways by preventing the phosphorylation and activation of STATs.
In biochemical assays, abrocitinib has selectivity for JAK1 over the other 3 JAK isoforms JAK2 (28-fold), JAK3 (>340-fold) and tyrosine kinase 2 (TYK2, 43-fold). In cellular settings, it preferentially inhibits cytokine-induced STAT phosphorylation by signalling pairs involving JAK1, and spares signalling by JAK2/JAK2, or JAK2/TYK2 pairs. The relevance of selective enzymatic inhibition of specific JAK enzymes to clinical effect is not currently known.
Pharmacodynamic effects
Clinical biomarkers
Treatment with abrocitinib was associated with dose-dependent reduction in serum biomarkers of inflammation in atopic dermatitis [interleukin-31 (IL-31), interleukin-22 (IL-22), eosinophil count, and thymus and activation-regulated chemokine (TARC)], JAK1 signalling [natural killer (NK) cell count and interferon gamma-induced protein 10 (IP-10)] or both [high sensitivity C-reactive protein (hsCRP)]. These changes were reversible after treatment discontinuation.
Mean absolute lymphocyte count increased by 2 weeks after starting treatment with abrocitinib and returned to baseline by Month 9 of treatment. Most patients maintained an ALC within the reference range. Treatment with abrocitinib was associated with a dose-related increase in B cell counts and a dose-related decrease in NK cell counts. The clinical significance of these changes in B cell and NK cell counts is unknown.
Cardiac electrophysiology
The effect of abrocitinib on the QTc interval was examined in subjects who received a single supratherapeutic dose of abrocitinib 600 mg in a placebo- and positive-controlled thorough QT study. A concentration-dependent QTc prolonging effect of abrocitinib was seen; the mean (90% confidence interval) for the increase in QTc interval was 6.0 (4.52, 7.49) msec, indicating the lack of a clinically relevant effect of abrocitinib on QTc interval at the dose tested.
Clinical efficacy and safety
The efficacy and safety of abrocitinib as monotherapy and in combination with background medicated topical therapies over 12-16 weeks were evaluated in 1 616 patients in 3 pivotal Phase 3 randomised, double-blind, placebo-controlled studies (MONO-1, MONO-2, and COMPARE). In addition, the efficacy and safety of abrocitinib in monotherapy over 52 weeks (with the option of rescue treatment in flaring patients) was evaluated in 1 233 patients in a Phase 3 induction, randomised withdrawal, double-blind, placebo-controlled study (REGIMEN). The patients in these 4 studies were 12 years of age and older with moderate-to-severe atopic dermatitis as defined by Investigator’s Global Assessment (IGA) score ≥3, Eczema Area and Severity Index (EASI) score ≥16, BSA involvement ≥10%, and Peak Pruritus Numerical Rating Scale (PP-NRS) ≥4 at baseline prior to randomisation. Patients who had a prior inadequate response or for whom topical treatments were medically unadvisable, or who had received systemic therapies were eligible for inclusion. All patients who completed the parent studies were eligible to enrol into the long-term extension study EXTEND.
Baseline characteristics
In the placebo-controlled studies (MONO-1, MONO-2, COMPARE) and the open-label induction, randomised withdrawal study (REGIMEN), across all treatment groups 41.4% to 51.1% were female, 59.3% to 77.8% were Caucasian, 15.0% to 33.0% were Asian and 4.1% to 8.3% were Black, and the mean age was 32.1 to 37.7 years. A total of 134 patients 65 years of age and older were enrolled in these studies. In these studies, 32.2% to 40.8% had a baseline IGA of 4 (severe atopic dermatitis), and 41.4% to 59.5% of patients had received prior systemic treatment for atopic dermatitis. The baseline mean EASI score ranged from 28.5 to 30.9, the baseline PP-NRS ranged from 7.0 to 7.3 and the baseline Dermatology Life Quality Index (DLQI) ranged from 14.4 to 16.0.
Clinical response
12-week monotherapy (MONO-1, MONO-2) and 16-week combination therapy (COMPARE) studies
A significantly larger proportion of patients achieved both primary endpoints IGA 0 or 1 and/or EASI-75 with 100 mg or 200 mg once daily abrocitinib compared with placebo at Week 12 or Week 16 (see Table 3 and Table 4).
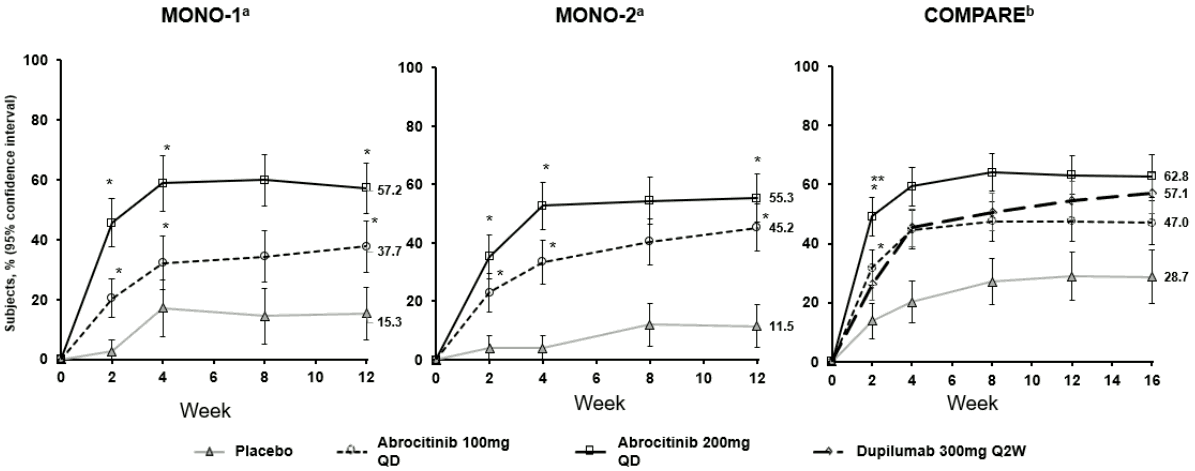
A significantly greater proportion of patients achieved at least a PP-NRS 4-point improvement with 100 mg or 200 mg once daily abrocitinib compared with placebo. This improvement was observed as early as Week 2 and persisted through Week 12 (Figure 1).
In the COMPARE study, superiority of abrocitinib 200 mg compared with dupilumab at Week 2 was demonstrated for the proportion of patients achieving at least a PP-NRS 4-point improvement with significantly higher itch responses seen as early as Day 4 after the first dose.
Treatment effects in subgroups (e.g. weight, age, sex, race and prior systemic immunosuppressant treatment) in MONO-1, MONO-2 and COMPARE were consistent with the results in the overall study population.
Table 3. Efficacy results of abrocitinib in monotherapy at Week 12:
| MONO-1d | MONO-2d | |||||
|---|---|---|---|---|---|---|
| Week 12 | Week 12 | |||||
| Abrocitinib monotherapy | PBO N=77 | Abrocitinib monotherapy | PBO N=78 | |||
| 200 mg QD N=154 | 100 mg QD N=156 | 200 mg QD N=155 | 100 mg QD N=158 | |||
| % Responders (95% CI) | ||||||
| IGA 0 or 1a | 43.8e (35.9, 51.7) | 23.7e (17.0, 30.4) | 7.9 (1.8, 14.0) | 38.1e (30.4, 45.7) | 28.4e (21.3, 35.5) | 9.1 (2.7, 15.5) |
| EASI-75b | 62.7e (55.1, 70.4) | 39.7e (32.1, 47.4) | 11.8 (4.6, 19.1) | 61.0e (53.3, 68.7) | 44.5e (36.7, 52.3) | 10.4 (3.6, 17.2) |
| PP-NRS4c | 57.2e (48.8, 65.6) | 37.7e (29.2, 46.3) | 15.3 (6.6, 24.0) | 55.3e (47.2, 63.5) | 45.2e (37.1, 53.3) | 11.5 (4.1, 19.0) |
Abbreviations: CI=confidence interval; EASI=Eczema Area and Severity Index; IGA=Investigator Global Assessment; N=number of patients randomised; PBO=placebo; PP-NRS=Peak Pruritus Numerical Rating Scale; QD=once daily.
a IGA responders were patients with IGA score of clear (0) or almost clear (1) (on a 5-point scale) and a reduction from baseline of ≥2 points.
b EASI-75 responders were patients with ≥75% improvement in EASI from baseline.
c PP-NRS4 responders were patients with ≥4-point improvement in PP-NRS from baseline.
d Abrocitinib used as monotherapy.
e Statistically significant with adjustment for multiplicity versus placebo
Table 4. Efficacy results of abrocitinib in combination with topical therapy at Week 12 and Week 16:
| COMPAREd | ||||||||
|---|---|---|---|---|---|---|---|---|
| Week 12 | Week 16 | |||||||
| Abrocitinib + topicals | PBO + topicals N=131 | DUP + topicals N=243 | Abrocitinib + topicals | PBO + topicals N=131 | DUP + topicals N=243 | |||
| 200 mg QD N=226 | 100 mg QD N=238 | 200 mg QD N=226 | 100 mg QD N=238 | |||||
| % Responders (95% CI) | ||||||||
| IGA 0 or 1a | 48.4e (41.8, 55.0) | 36.6e (30.4, 42.8) | 14.0 (8.0, 19.9) | 36.5 (30.4, 42.6) | 47.5e (40.9, 54.1) | 34.8e (28.6, 40.9) | 12.9 (7.0, 18.8) | 38.8 (32.5, 45.1) |
| EASI-75b | 70.3e (64.3, 76.4) | 58.7e (52.4, 65.0) | 27.1 (19.5, 34.8) | 58.1 (51.9, 64.3) | 71.0e (65.1, 77.0) | 60.3e (53.9, 66.6) | 30.6 (22.5, 38.8) | 65.5 (59.4, 71.6) |
| PP-NRS4c | 63.1 (56.7, 69.6) | 47.5 (40.9, 54.1) | 28.9 (20.8, 37.0) | 54.5 (47.9, 61.0) | 62.8 (55.6, 70.0) | 47.0 (39.5, 54.6) | 28.7 (19.6, 37.9) | 57.1 (50.1, 64.2) |
Abbreviations: CI=confidence interval; DUP=Dupilumab; EASI=Eczema Area and Severity Index; IGA=Investigator Global Assessment; N=number of patients randomised; PBO=placebo; PP-NRS=Peak Pruritus Numerical Rating Scale; QD=once daily.
a IGA responders were patients with IGA score of clear (0) or almost clear (1) (on a 5-point scale) and a reduction from baseline of ≥2 points.
b EASI-75 responders were patients with ≥75% improvement in EASI from baseline.
c PP-NRS4 responders were patients with ≥4-point improvement in PP-NRS from baseline.
d Abrocitinib used in combination with topical therapy.
e Statistically significant with adjustment for multiplicity versus placebo.
The proportion of patients who achieved PP-NRS4 over time in studies MONO-1, MONO-2 and COMPARE are shown in Figure 1.
Figure 1. Proportion of patients who achieved PP-NRS4 over time in MONO-1, MONO-2 and COMPARE:
Abbreviations: PP-NRS=Peak Pruritus Numerical Rating Scale; QD=once daily; Q2W=every 2 weeks.
PP-NRS4 responders were patients with ≥4-point improvement in PP-NRS from baseline.
a Abrocitinib used as monotherapy.
b Abrocitinib used in combination with medicated topical therapy.
* Statistically significant with adjustment for multiplicity versus placebo.
** Statistically significant with adjustment for multiplicity versus dupilumab.
Health-related outcomes
In both monotherapy studies (MONO-1 and MONO-2) and in the combination therapy study (COMPARE), abrocitinib significantly improved patient-reported outcomes, including itch, sleep (SCORAD Sleep VAS), AD symptoms (POEM), quality of life (DLQI) and symptoms of anxiety and depression (HADS) that were uncorrected for multiplicity, at 12 weeks compared to placebo (see Table 5).
Table 5. Patient-reported outcomes results of abrocitinib monotherapy and in combination with topical therapy at Week 12:
| Monotherapy | Combination therapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MONO-1 | MONO-2 | COMPARE | |||||||
| 200 mg QD | 100 mg QD | PBO | 200 mg QD | 100 mg QD | PBO | 200 mg QD + topicals | 100 mg QD + topicals | PBO + topicals | |
| N | 154 | 156 | 77 | 155 | 158 | 78 | 226 | 238 | 131 |
| SCORAD Sleep VAS, change from baseline (95% CI) | -3.7* (-4.2, -3.3) | -2.9* (-3.4, -2.5) | -1.6 (-2.2, -1.0) | -3.8* (-4.2, -3.4) | -3.0* (-3.4, -2.6) | -2.1 (-2.7, -1.5) | -4.6* (-4.9, -4.3) | -3.7* (-4.0, -3.4) | -2.4 (-2.8, -2.0) |
| DLQI ≥4-point improvement, % responders | 72.6%* | 67.2%* | 43.6% | 78.1%* | 73.3%* | 32.3% | 86.4%* | 74.7%* | 56.5% |
| POEM, change from baseline (95% CI) | -10.6* (-11.8, -9.4) | -6.8* (-8.0, -5.6) | -3.7 (-5.5, -1.9) | -11.0* (-12.1, -9.8) | -8.7* (-9.9, -7.5) | -3.6 (-5.3, -1.9) | -12.6* (-13.6, -11.7) | -9.6* (-10.5, -8.6) | -5.1 (-6.3, -3.9) |
| HADS Anxiety, change from baseline (95% CI) | -2.1* (-2.5, -1.6) | -1.6 (-2.0, -1.1) | -1.0 (-1.7, -0.4) | -1.7* (-2.2, -1.2) | -1.6* (-2.1, -1.1) | -0.6 (-1.3, 0.2) | -1.6* (-2.0, -1.2) | -1.2* (-1.5, -0.8) | -0.4 (-0.9, 0.1) |
| HADS Depression, change from baseline (95% CI) | -1.8* (-2.2, -1.4) | -1.4* (-1.8, -0.9) | -0.2 (-0.8, 0.4) | -1.4* (-1.8, -1.0) | -1.0* (-1.5, -0.6) | 0.3 (-0.3, 0.9) | -1.6* (-1.9, -1.2) | -1.3* (-1.6, -0.9) | -0.3 (-0.7, 0.2) |
CI=confidence interval; DLQI=Dermatology Life Quality Index; HADS=Hospital Anxiety and Depression Scale; N=number of patients randomised; PBO=placebo; POEM=Patient-Oriented Eczema Measure; QD=once daily; SCORAD=SCORing for AD; VAS=visual analog scale.
* Statistically significant without adjusting for multiplicity
Open-label induction, randomised withdrawal study (REGIMEN)
A total of 1 233 patients received open-label abrocitinib 200 mg once daily in the 12-week run-in phase. Among these patients, 798 patients (64.7%) met responder criteria (defined as achieving IGA [0 or 1] response and EASI-75) and were randomised to placebo (267 patients), abrocitinib 100 mg once daily (265 patients) or abrocitinib 200 mg once daily (266 patients).
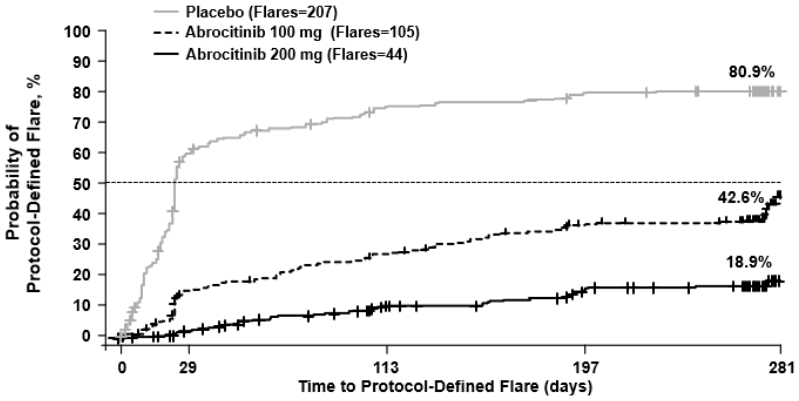
Continuous treatment (200 mg continuous) and induction-maintenance treatment (200 mg for 12 weeks followed by 100 mg) prevented flare with 81.1% and 57.4% probability, respectively, versus 19.1% among patients who withdrew treatment (randomised to placebo) after 12 weeks of induction. Three-hundred fifty-one (351) patients including 16.2% of 200 mg, 39.2% of 100 mg and 76.4% of placebo patients received rescue medication of 200 mg abrocitinib in combination with topical therapy.
Figure 2. Time to protocol-defined flare:
Abrocitinib used as monotherapy.
Protocol-defined flare = A loss of at least 50% of the EASI response at Week 12 and an IGA score of 2 or higher.
Multiplicity-controlled p<0.0001 200 mg versus placebo; 100 mg versus placebo; 200 mg versus 100 mg.
Long-term efficacy
Eligible patients who completed the full treatment period of a qualifying parent study (e.g. MONO-1, MONO-2, COMPARE, REGIMEN) were considered for enrolment in the long-term extension study EXTEND. In EXTEND, patients received abrocitinib with or without background medicated topical therapy. Patients who were previously randomised to medicinal product 100 mg or 200 mg once daily in parent studies continued the same dose in EXTEND as in the parent study. In EXTEND, patients received double-blind treatment until the parent study was completed, after which patients received single-blind treatment (treatment assignment disclosed to the investigators but not to the patients).
Among patients who achieved response after 12 weeks of treatment and entered EXTEND, the majority of patients maintained their response at Week 96 of cumulative treatment for both doses of abrocitinib [64% and 72% for IGA (0 or 1) response, 87% and 90% for EASI-75, and 75% and 80% for PP-NRS4 with 100 mg once daily and 200 mg once daily, respectively].
Among patients who did not achieve response after 12 weeks of treatment and entered EXTEND, a proportion of patients achieved late-onset response by Week 24 (from baseline) of continued treatment with abrocitinib [25% and 29% for IGA (0 or 1) response, and 50% and 57% for EASI-75 with 100 mg once daily and 200 mg once daily, respectively]. Patients who achieved partial response at Week 12 were more likely than those with no response at Week 12 to achieve treatment benefit at Week 24.
Patients who received dupilumab in the COMPARE study and subsequently entered EXTEND were randomised to either 100 mg or 200 mg of abrocitinib once daily upon entering EXTEND. Among non-responders to dupilumab, a substantial proportion of patients achieved response 12 weeks after switching to abrocitinib [34% and 47% for IGA (0 or 1) response, and 68% and 80% for EASI-75 with 100 mg once daily or 200 mg once daily, respectively].
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with abrocitinib in one or more subsets of the paediatric population in the treatment of atopic dermatitis (see section 4.2 for information on paediatric use).
The efficacy and safety of 12 weeks of abrocitinib monotherapy were evaluated in 2 Phase 3 randomised, double-blind, placebo-controlled studies (MONO-1, MONO-2) which included 124 patients who were 12 to less than 18 years of age. The efficacy and safety of abrocitinib monotherapy over 52 weeks (with the option of rescue treatment for flaring patients) were also evaluated in an open-label induction, randomised withdrawal study (REGIMEN), which included 246 patients who were 12 to less than 18 years of age. In these studies, the results in the adolescent subgroup were consistent with the results in the overall study population.
The efficacy and safety of 12 weeks of abrocitinib in combination with background medicated topical therapy were evaluated in the Phase 3 randomised, double-blind, placebo-controlled study TEEN. The study included 287 patients who were 12 to less than 18 years of age with moderate-to-severe atopic dermatitis as defined by IGA score ≥3, EASI score ≥16, BSA involvement ≥10%, and PP-NRS ≥4 at the baseline visit prior to randomisation. Patients who had a prior inadequate response or who had received systemic therapy, were eligible for inclusion.
Baseline characteristics
In TEEN, across all treatment groups 49.1% were female, 56.1% were Caucasian, 33.0% were Asian and 6.0% were Black patients. The median age was 15 years and the proportion of patients with severe atopic dermatitis (IGA of 4) was 38.6%.
Results of 12-week abrocitinib treatment in adolescents in pooled MONO-1 and MONO-2, and the TEEN study are shown in Table 6.
Table 6. Adolescent efficacy results at Week 12 in pooled MONO-1 and MONO-2, and in TEEN:
| Pooled MONO-1 and MONO-2 | TEENd | |||||
|---|---|---|---|---|---|---|
| Abrocitinib 200 mg QD | Abrocitinib 100 mg QD | Placebo | Abrocitinib 200 mg QD | Abrocitinib 100 mg QD | Placebo | |
| IGA 0 or 1a | ||||||
| N | 48 | 50 | 23 | 93 | 89 | 94 |
| % | 31.3 | 22.0 | 8.7 | 46.2e | 41.6e | 24.5 |
| 95% CI | (18.1, 44.4) | (10.5, 33.5) | (0.0, 20.2) | (36.1, 56.4) | (31.3, 51.8) | (15.8, 33.2) |
| EASI-75b | ||||||
| N | 48 | 50 | 23 | 93 | 89 | 94 |
| % | 56.3 | 44.0 | 8.7 | 72.0e | 68.5e | 41.5 |
| 95% CI | (42.2, 70.3) | (30.2, 57.8) | (0.0, 20.2) | (62.9, 81.2) | (58.9, 78.2) | (31.5, 51.4) |
| PP-NRS4c | ||||||
| N | 36 | 42 | 22 | 74 | 76 | 84 |
| % | 61.1 | 28.6 | 9.1 | 55.4e | 52.6 | 29.8 |
| 95% CI | (45.2, 77.0) | (14.9, 42.2) | (0.0, 21.1) | (44.1, 66.7) | (41.4, 63.9) | (20.0, 39.5) |
Abbreviations: CI=confidence interval; EASI=Eczema Area and Severity Index; IGA=Investigator Global Assessment; N=number of evaluable patients; PP-NRS=Peak Pruritus Numerical Rating Scale; QD=once daily.
a IGA responders were patients with IGA score of clear (0) or almost clear (1) (on a 5-point scale) and a reduction from baseline of ≥2 points.
b EASI-75 responders were patients with ≥75% improvement in EASI from baseline.
c PP-NRS4 responders were patients with ≥4-point improvement in PP-NRS from baseline.
d Abrocitinib used in combination with medicated topical therapy.
e Statistically significant with adjustment for multiplicity versus placebo.
Among adolescent patients who achieved response after 12 weeks of treatment and entered long-term extension study EXTEND, the majority of patients maintained their response at Week 96 of cumulative treatment for both doses of abrocitinib [62% and 78% for IGA (0 or 1) response, 89% and 93% for EASI-75, and 77% and 76% for PP-NRS4 with 100 mg and 200 mg once daily, respectively].
Among adolescent patients who did not achieve response after 12 weeks of treatment and entered EXTEND, a proportion of patients achieved late-onset response by Week 24 (from baseline) of continued treatment with both doses of abrocitinib [34% and 28% for IGA (0 or 1) response, and 41% and 55% for EASI-75 with 100 mg and 200 mg once daily, respectively].
5.2. Pharmacokinetic properties
Absorption
Abrocitinib is well-absorbed with over 91% extent of oral absorption and absolute oral bioavailability of approximately 60%. The oral absorption of abrocitinib is rapid and peak plasma concentrations are reached within 1 hour. Steady-state plasma concentrations of abrocitinib are achieved within 48 hours after once daily administration. Both Cmax and AUC of abrocitinib increased dose proportionally up to 200 mg. Co-administration of abrocitinib with a high-fat meal had no clinically relevant effect on abrocitinib exposures (AUC and Cmax increased by approximately 26% and 29%, respectively, and Tmax was prolonged by 2 hours). In clinical studies, abrocitinib was administered without regard to food (see section 4.2).
Distribution
After intravenous administration, the volume of distribution of abrocitinib is about 100 L. Approximately 64%, 37% and 29% of circulating abrocitinib and its active metabolites M1 and M2, respectively, are bound to plasma proteins. Abrocitinib and its active metabolites distribute equally between red blood cells and plasma.
Biotransformation
The in vitro metabolism of abrocitinib is mediated by multiple CYP enzymes, CYP2C19 (~53%), CYP2C9 (~30%), CYP3A4 (~11%) and CYP2B6 (~6%). In a human radiolabelled study, abrocitinib was the most prevalent circulating species, with mainly 3 polar mono-hydroxylated metabolites identified as M1 (3-hydroxypropyl), M2 (2-hydroxypropyl) and M4 (pyrrolidinone pyrimidine). At steady state, M2 and M4 are major metabolites and M1 is a minor metabolite. Of the 3 metabolites in circulation, M1 and M2 have similar JAK inhibitory profiles as abrocitinib, while M4 was pharmacologically inactive. The pharmacologic activity of abrocitinib is attributable to the unbound exposures of parent molecule (~60%) as well as M1 (~10%) and M2 (~30%) in systemic circulation. The sum of unbound exposures of abrocitinib, M1 and M2, each expressed in molar units and adjusted for relative potencies, is referred to as the abrocitinib active moiety.
No clinically significant effects of abrocitinib were observed in interaction studies with substrates of BCRP and OAT3 (e.g. rosuvastatin), MATE1/2K (e.g. metformin), CYP3A4 (e.g. midazolam), and CYP2B6 (e.g. efavirenz).
Elimination
The elimination half-life of abrocitinib is about 5 hours. Abrocitinib is eliminated primarily by metabolic clearance mechanisms, with less than 1% of the dose excreted in urine as unchanged active substance. The metabolites of abrocitinib, M1, M2 and M4 are excreted predominantly in urine, and are substrates of OAT3 transporter.
Special populations
Body weight, gender, genotype, race and age
Body weight, gender, CYP2C19/2C9 genotype, race and age did not have a clinically meaningful effect on abrocitinib exposure (see section 4.2).
Adolescents (≥12 to <18 years)
Based on population pharmacokinetic analysis, there was no clinically relevant difference in mean abrocitinib steady-state exposures in adolescent patients compared to adults at their typical body weights.
Paediatric (<12 years)
Interaction studies have been performed in adults only. The pharmacokinetics of abrocitinib in children under 12 years of age have not yet been established (see section 4.2).
Renal impairment
In a renal impairment study, patients with severe (eGFR <30 mL/min) and moderate (eGFR 30 to <60 mL/min) renal impairment had approximately 191% and 110% increase in active moiety AUCinf, respectively, compared to patients with normal renal function (eGFR ≥90 mL/min) (see section 4.2). Pharmacokinetics of abrocitinib have not been determined in patients with mild renal impairment, however, based on the results observed in other groups, an increase of up to 70% in active moiety exposure is expected in patients with mild renal impairment (eGFR 60 to <90 mL/min). The increase of up to 70% is not clinically meaningful as the efficacy and safety of abrocitinib in atopic dermatitis patients with mild renal impairment (n=756) was comparable to the overall population in Phase 2 and 3 clinical studies. The eGFR in individual patients was estimated using Modification of Diet in Renal Disease (MDRD) formula.
Abrocitinib has not been studied in patients with ESRD on renal replacement therapy (see section 4.2). In Phase 3 clinical studies, abrocitinib was not evaluated in patients with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
Hepatic impairment
Patients with mild (Child Pugh A) and moderate (Child Pugh B) hepatic impairment had approximately 4% decrease and 15% increase in active moiety AUCinf, respectively, compared to patients with normal hepatic function. These changes are not clinically significant, and no dose adjustment is required in patients with mild or moderate hepatic impairment (see section 4.2). In clinical studies, abrocitinib was not evaluated in patients with severe (Child Pugh C) hepatic impairment (see section 4.3), or in patients screened positive for active hepatitis B or hepatitis C (see section 4.4).
5.3. Preclinical safety data
General toxicity
Decreased lymphocyte counts and decreased size and/or lymphoid cellularity of organs/tissues of the immune and haematopoietic systems were observed in nonclinical studies and were attributed to the pharmacological properties (JAK inhibition) of abrocitinib.
In toxicity studies of up to 1 month of abrocitinib dosing in rats at an age comparable to adolescent human age of ≥12 years, a microscopic bone dystrophy finding, considered transient and reversible, was noted, and exposure margins at which no bone finding was noted were 5.7 to 6.1 times the human AUC at the maximum recommended human dose (MRHD) of 200 mg. No bone findings were observed in rats at any dose in the 6-month toxicity study (up to 25 times the human AUC at the MRHD of 200 mg) or in any of the toxicity studies in cynomolgus monkeys (comparable to human age of ≥8 years; up to 30 times the human AUC at the MRHD of 200 mg).
Genotoxicity
Abrocitinib was not mutagenic in the bacterial mutagenicity assay (Ames assay). It was not aneugenic or clastogenic based on the results of the in vivo rat bone marrow micronucleus assay.
Carcinogenicity
No evidence of tumorigenicity was observed in the 6-month Tg.rasH2 mice administered abrocitinib at oral doses up to 75 mg/kg/day and 60 mg/kg/day in female and male mice, respectively. In the 2-year carcinogenicity study, higher incidence of benign thymoma was noted in female rats at the lowest dose tested. Thus, a lowest observed adverse effect level (LOAEL) is set in females at exposures equal to 0.6 times the human AUC at the MRHD of 200 mg. In males the no observed adverse effect level (NOAEL) was set at exposures equal to 13 times the human AUC at the MRHD of 200 mg. The human relevance of benign thymoma is unknown.
Reproductive and developmental toxicity
Abrocitinib had no effects on male fertility or spermatogenesis. Abrocitinib resulted in effects on female fertility (lower fertility index, corpora lutea, implantation sites and post-implantation loss), but no fertility effects were noted at exposures equal to 1.9 times the human AUC at the MRHD of 200 mg. The effects reversed 1 month after cessation of treatment.
No foetal malformations were observed in embryo-foetal development studies in rats or rabbits. In an embryo-foetal development study in pregnant rabbits, effects on embryo-foetal survival were noted at the lowest dose tested with exposures equal to 0.14 times the unbound human AUC at the MRHD of 200 mg. Increased litter incidences of unossified hindlimb phalanges and tarsals and forelimb phalanges were observed with effects on forelimb phalanges noted at exposures equal to 0.14 times the unbound human AUC at the MRHD of 200 mg.
In an embryo-foetal development study in pregnant rats, while increased embryo-foetal lethality was noted, none was observed at exposures equal to 10 times the human AUC at the MRHD of 200 mg. Increased incidence of skeletal variations of short 13th ribs, reduced ventral processes, thickened ribs, and unossified metatarsals were noted in the foetuses, but none were observed at exposures equal to 2.3 times the human AUC at the MRHD of 200 mg.
In a pre- and postnatal development study in pregnant rats, dams had dystocia with prolonged parturition, offspring had lower body weights and lower postnatal survival. No maternal or developmental toxicity was observed in either dams or offspring at exposures equal to 2.3 times the human AUC at the MRHD of 200 mg.
Administration of abrocitinib to juvenile rats beginning on postnatal Day 10 (comparable to a 3-month-old human infant) resulted in adverse microscopic and macroscopic bone findings, including malrotated paws, fractures, and/or femoral head abnormalities at exposures ≥0.8 times the human AUC at the MRHD of 200 mg. Administration of abrocitinib to juvenile rats beginning on postnatal Day 21 and older (comparable to a 2-year-old human and older) was not associated with microscopic or macroscopic bone finding.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.