COSENTYX Solution for injection Ref.[8526] Active ingredients: Secukinumab
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, interleukin inhibitors
ATC code: L04AC10
h2 Mechanism of action
Secukinumab is a fully human IgG1/κ monoclonal antibody that selectively binds to and neutralises the proinflammatory cytokine interleukin-17A (IL-17A). Secukinumab works by targeting IL-17A and inhibiting its interaction with the IL-17 receptor, which is expressed on various cell types including keratinocytes. As a result, secukinumab inhibits the release of proinflammatory cytokines, chemokines and mediators of tissue damage and reduces IL-17A-mediated contributions to autoimmune and inflammatory diseases. Clinically relevant levels of secukinumab reach the skin and reduce local inflammatory markers. As a direct consequence treatment with secukinumab reduces erythema, induration and desquamation present in plaque psoriasis lesions.
IL-17A is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. IL-17A plays a key role in the pathogenesis of plaque psoriasis, hidradenitis suppurativa, psoriatic arthritis and axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis) and is up-regulated in lesional skin in contrast to non-lesional skin of plaque psoriasis patients and in synovial tissue of psoriatic arthritis patients. IL-17A is also upregulated in hidradenitis suppurativa lesions and increased IL-17A serum levels have been observed in affected patients. The frequency of IL-17-producing cells was also significantly higher in the subchondral bone marrow of facet joints from patients with ankylosing spondylitis. Increased numbers of IL-17A producing lymphocytes have also been found in patients with non-radiographic axial spondyloarthritis. Inhibition of IL-17A was shown to be effective in the treatment of ankylosing spondylitis, thus establishing the key role of this cytokine in axial spondyloarthritis.
Pharmacodynamic effects
Serum levels of total IL-17A (free and secukinumab-bound IL-17A) are initially increased in patients receiving secukinumab. This is followed by a slow decrease due to reduced clearance of secukinumab- bound IL-17A, indicating that secukinumab selectively captures free IL-17A, which plays a key role in the pathogenesis of plaque psoriasis.
In a study with secukinumab, infiltrating epidermal neutrophils and various neutrophil-associated markers that are increased in lesional skin of plaque psoriasis patients were significantly reduced after one to two weeks of treatment.
Secukinumab has been shown to lower (within 1 to 2 weeks of treatment) levels of C-reactive protein, which is a marker of inflammation.
Clinical efficacy and safety
Adult plaque psoriasis
The safety and efficacy of secukinumab were assessed in four randomised, double-blind, placebo-controlled phase III studies in patients with moderate to severe plaque psoriasis who were candidates for phototherapy or systemic therapy [ERASURE, FIXTURE, FEATURE, JUNCTURE]. The efficacy and safety of secukinumab 150 mg and 300 mg were evaluated versus either placebo or etanercept. In addition, one study assessed a chronic treatment regimen versus a "retreatment as needed" regimen [SCULPTURE].
Of the 2 403 patients who were included in the placebo-controlled studies, 79% were biologic-naive, 45% were non-biologic failures and 8% were biologic failures (6% were anti-TNF failures, and 2% were anti-p40 failures). Approximately 15 to 25% of patients in phase III studies had psoriatic arthritis (PsA) at baseline.
Psoriasis study 1 (ERASURE) evaluated 738 patients. Patients randomised to secukinumab received 150 mg or 300 mg doses at weeks 0, 1, 2, 3 and 4, followed by the same dose every month. Psoriasis study 2 (FIXTURE) evaluated 1 306 patients. Patients randomised to secukinumab received 150 mg or 300 mg doses at weeks 0, 1, 2, 3 and 4, followed by the same dose every month. Patients randomised to etanercept received 50 mg doses twice per week for 12 weeks followed by 50 mg every week. In both study 1 and study 2, patients randomised to receive placebo who were non-responders at week 12 then crossed over to receive secukinumab (either 150 mg or 300 mg) at weeks 12, 13, 14, and 15, followed by the same dose every month starting at week 16. All patients were followed for up to 52 weeks following first administration of study treatment.
Psoriasis study 3 (FEATURE) evaluated 177 patients using a pre-filled syringe compared with placebo after 12 weeks of treatment to assess the safety, tolerability, and usability of secukinumab self-administration via the pre-filled syringe. Psoriasis study 4 (JUNCTURE) evaluated 182 patients using a pre-filled pen compared with placebo after 12 weeks of treatment to assess the safety, tolerability, and usability of secukinumab self-administration via the pre-filled pen. In both study 3 and study 4, patients randomised to secukinumab received 150 mg or 300 mg doses at weeks 0, 1, 2, 3 and 4, followed by the same dose every month. Patients were also randomised to receive placebo at weeks 0, 1, 2, 3 and 4, followed by the same dose every month.
Psoriasis study 5 (SCULPTURE) evaluated 966 patients. All patients received secukinumab 150 mg or 300 mg doses at weeks 0, 1, 2, 3, 4, 8 and 12 and then were randomised to receive either a maintenance regimen of the same dose every month starting at week 12 or a "retreatment as needed" regimen of the same dose. Patients randomised to "retreatment as needed" did not achieve adequate maintenance of response and therefore a fixed monthly maintenance regimen is recommended.
The co-primary endpoints in the placebo and active-controlled studies were the proportion of patients who achieved a PASI 75 response and IGA mod 2011 "clear" or "almost clear" response versus placebo at week 12 (see Tables 4 and 5). The 300 mg dose provided improved skin clearance particularly for "clear" or "almost clear" skin across the efficacy endpoints of PASI 90, PASI 100, and IGA mod 2011 0 or 1 response across all studies with peak effects seen at week 16, therefore this dose is recommended.
Table 4. Summary of PASI 50/75/90/100 & IGA⃰ mod 2011 "clear" or "almost clear" clinical response in psoriasis studies 1, 3 and 4 (ERASURE, FEATURE and JUNCTURE):
| Week 12 | Week 16 | Week 52 | |||||
|---|---|---|---|---|---|---|---|
| Placebo | 150 mg | 300 mg | 150 mg | 300 mg | 150 mg | 300 mg | |
| Study 1 | |||||||
| Number of patients | 246 | 244 | 245 | 244 | 245 | 244 | 245 |
| PASI 50 response n (%) | 22 (8.9%) | 203 (83.5%) | 222 (90.6%) | 212 (87.2%) | 224 (91.4%) | 187 (77%) | 207 (84.5%) |
| PASI 75 response n (%) | 11 (4.5%) | 174 (71.6%)** | 200 (81.6%)** | 188 (77.4%) | 211 (86.1%) | 146 (60.1%) | 182 (74.3%) |
| PASI 90 response n (%) | 3 (1.2%) | 95 (39.1%)** | 145 (59.2%)** | 130 (53.5%) | 171 (69.8%) | 88 (36.2%) | 147 (60.0%) |
| PASI 100 response n (%) | 2 (0.8%) | 31 (12.8%) | 70 (28.6%) | 51 (21.0%) | 102 (41.6%) | 49 (20.2%) | 96 (39.2%) |
| IGA mod 2011 "clear" or "almost clear" response n (%) | 6 (2.40%) | 125 (51.2%)** | 160 (65.3%)** | 142 (58.2%) | 180 (73.5%) | 101 (41.4%) | 148 (60.4%) |
| Study 3 | |||||||
| Number of patients | 59 | 59 | 58 | - | - | - | - |
| PASI 50 response n (%) | 3 (5.1%) | 51 (86.4%) | 51 (87.9%) | - | - | - | - |
| PASI 75 response n (%) | 0 (0.0%) | 41 (69.5%)** | 44 (75.9%)** | - | - | - | - |
| PASI 90 response n (%) | 0 (0.0%) | 27 (45.8%) | 35 (60.3%) | - | - | - | - |
| PASI 100 response n (%) | 0 (0.0%) | 5 (8.5%) | 25 (43.1%) | - | - | - | - |
| IGA mod 2011 "clear" or "almost clear" response n (%) | 0 (0.0%) | 31 (52.5%)** | 40 (69.0%)** | - | - | - | - |
| Study 4 | |||||||
| Number of patients | 61 | 60 | 60 | - | - | - | - |
| PASI 50 response n (%) | 5 (8.2%) | 48 (80.0%) | 58 (96.7%) | - | - | - | - |
| PASI 75 response n (%) | 2 (3.3%) | 43 (71.7%)** | 52 (86.7%)** | - | - | - | - |
| PASI 90 response n (%) | 0 (0.0%) | 24 (40.0%) | 33 (55.0%) | - | - | - | - |
| PASI 100 response n(%) | 0 (0.0%) | 10 (16.7%) | 16 (26.7%) | - | - | - | - |
| IGA mod 2011 "clear" or "almost clear" response n (%) | 0 (0.0%) | 32 (53.3%)** | 44 (73.3%)** | - | - | - | - |
* The IGA mod 2011 is a 5-category scale including "0 = clear", "1 = almost clear", "2 = mild", "3 = moderate" or "4 = severe", indicating the physician's overall assessment of the psoriasis severity focusing on induration, erythema and scaling. Treatment success of "clear" or "almost clear" consisted of no signs of psoriasis or normal to pink colouration of lesions, no thickening of the plaque and none to minimal focal scaling.
** p-values versus placebo and adjusted for multiplicity: p<0.0001.
Table 5. Summary of clinical response on psoriasis study 2 (FIXTURE):
| Week 12 | Week 16 | Week 52 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 150 mg | 300 mg | Etanercept | 150 mg | 300 mg | Etanercept | 150 mg | 300 mg | Etanercept | |
| Number of patients | 324 | 327 | 323 | 323 | 327 | 323 | 323 | 327 | 323 | 323 |
| PASI 50 response n (%) | 49 (15.1%) | 266 (81.3%) | 296 (91.6%) | 226 (70.0%) | 290 (88.7%) | 302 (93.5%) | 257 (79.6%) | 249 (76.1%) | 274 (84.8%) | 234 (72.4%) |
| PASI 75 response n (%) | 16 (4.9%) | 219 (67.0%)** | 249 (77.1%)** | 142 (44.0%) | 247 (75.5%) | 280 (86.7%) | 189 (58.5%) | 215 (65.7%) | 254 (78.6%) | 179 (55.4%) |
| PASI 90 response n (%) | 5 (1.5%) | 137 (41.9%) | 175 (54.2%) | 67 (20.7%) | 176 (53.8%) | 234 (72.4%) | 101 (31.3%) | 147 (45.0%) | 210 (65.0%) | 108 (33.4%) |
| PASI 100 response n (%) | 0 (0%) | 47 (14.4%) | 78 (24.1%) | 14 (4.3%) | 84 (25.7%) | 119 (36.8%) | 24 (7.4%) | 65 (19.9%) | 117 (36.2%) | 32 (9.9%) |
| IGA mod 2011 "clear" or "almost clear" response n (%) | 9 (2.8%) | 167 (51.1%)** | 202 (62.5%)** | 88 (27.2%) | 200 (61.2%) | 244 (75.5%) | 127 (39.3%) | 168 (51.4%) | 219 (67.8%) | 120 (37.2%) |
** p-values versus etanercept: p=0.0250
In an additional psoriasis study (CLEAR) 676 patients were evaluated. Secukinumab 300 mg met the primary and secondary endpoints by showing superiority to ustekinumab based on PASI 90 response at week 16 (primary endpoint), speed of onset of PASI 75 response at week 4, and long-term PASI 90 response at week 52. Greater efficacy of secukinumab compared to ustekinumab for the endpoints PASI 75/90/100 and IGA mod 2011 0 or 1 response ("clear" or "almost clear") was observed early and continued through to week 52 (Table 6).
Table 6. Summary of clinical response on CLEAR study:
| Week 4 | Week 16 | Week 52 | ||||
|---|---|---|---|---|---|---|
| Secukinumab 300 mg | Ustekinumab* | Secukinumab 300 mg | Ustekinumab* | Secukinumab 300 mg | Ustekinumab* | |
| Number of patients | 334 | 335 | 334 | 335 | 334 | 335 |
| PASI 75 response n (%) | 166 (49.7%)** | 69 (20.6%) | 311 (93.1%) | 276 (82.4%) | 306 (91.6%) | 262 (78.2%) |
| PASI 90 response n (%) | 70 (21.0%) | 18 (5.4%) | 264 (79.0%)** | 192 (57.3%) | 250 (74.9%)*** | 203 (60.6%) |
| PASI 100 response n (%) | 14 (4.2%) | 3 (0.9%) | 148 (44.3%) | 95 (28.4%) | 150 (44.9%) | 123 (36.7%) |
| IGA mod 2011 "clear" or "almost clear" response n (%) | 128 (38.3%) | 41 (12.2%) | 278 (83.2%) | 226 (67.5%) | 261 (78.1%) | 213 (63.6%) |
* Patients treated with secukinumab received 300 mg doses at weeks 0, 1, 2 3 and 4, followed by the same dose every 4 weeks until week 52. Patients treated with ustekinumab received 45 mg or 90 mg at weeks 0 and 4, then every 12 weeks until week 52 (dosed by weight as per approved posology)
** p-values versus ustekinumab: p<0.0001 for primary endpoint of PASI 90 at week 16 and secondary endpoint of PASI 75 at week 4
*** p-values versus ustekinumab: p=0.0001 for secondary endpoint of PASI 90 at week 52
Secukinumab was efficacious in systemic treatment-naive, biologic-naive, biologic/anti-TNF-exposed and biologic/anti-TNF-failure patients. Improvements in PASI 75 in patients with concurrent psoriatic arthritis at baseline were similar to those in the overall plaque psoriasis population.
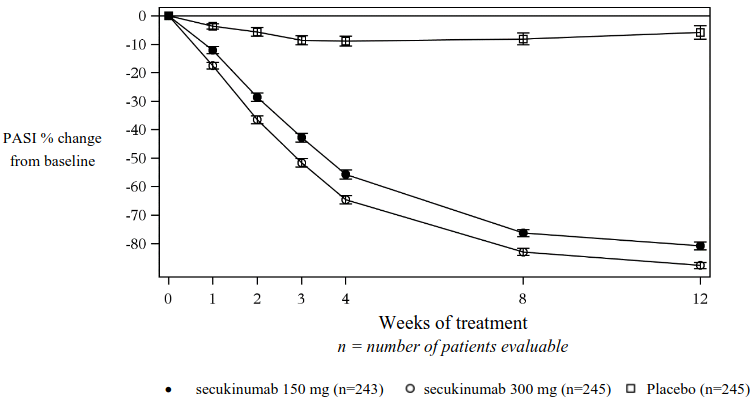
Secukinumab was associated with a fast onset of efficacy with a 50% reduction in mean PASI by week 3 for the 300 mg dose.
Figure 1. Time course of percentage change from baseline of mean PASI score in study 1 (ERASURE):
Specific locations/forms of plaque psoriasis
In two additional placebo-controlled studies, improvement was seen in both nail psoriasis (TRANSFIGURE, 198 patients) and palmoplantar plaque psoriasis (GESTURE, 205 patients). In the TRANSFIGURE study, secukinumab was superior to placebo at week 16 (46.1% for 300 mg, 38.4% for 150 mg and 11.7% for placebo) as assessed by significant improvement from baseline in the Nail Psoriasis Severity Index (NAPSI %) for patients with moderate to severe plaque psoriasis with nail involvement. In the GESTURE study, secukinumab was superior to placebo at week 16 (33.3% for 300 mg, 22.1% for 150 mg, and 1.5% for placebo) as assessed by significant improvement of ppIGA 0 or 1 response ("clear" or "almost clear") for patients with moderate to severe palmoplantar plaque psoriasis.
A placebo-controlled study evaluated 102 patients with moderate to severe scalp psoriasis, defined as having a Psoriasis Scalp Severity Index (PSSI) score of ≥12, an IGA mod 2011 scalp only score of 3 or greater and at least 30% of the scalp surface area affected. Secukinumab 300 mg was superior to placebo at week 12 as assessed by significant improvement from baseline in both the PSSI 90 response (52.9% versus 2.0%) and IGA mod 2011 0 or 1 scalp only response (56.9% versus 5.9%). Improvement in both endpoints was sustained for secukinumab patients who continued treatment through to week 24.
Quality of life/patient-reported outcomes
Statistically significant improvements at week 12 (studies 1-4) from baseline compared to placebo were demonstrated in the DLQI (Dermatology Life Quality Index). Mean decreases (improvements) in DLQI from baseline ranged from -10.4 to -11.6 with secukinumab 300 mg, from -7.7 to -10.1 with secukinumab 150 mg, versus -1.1 to -1.9 for placebo at week 12. These improvements were maintained for 52 weeks (studies 1 and 2).
Forty percent of the participants in studies 1 and 2 completed the Psoriasis Symptom Diary©. For the participants completing the diary in each of these studies, statistically significant improvements at week 12 from baseline compared to placebo in patient-reported signs and symptoms of itching, pain and scaling were demonstrated.
Statistically significant improvements at week 4 from baseline in patients treated with secukinumab compared to patients treated with ustekinumab (CLEAR) were demonstrated in the DLQI and these improvements were maintained for up to 52 weeks.
Statistically significant improvements in patient-reported signs and symptoms of itching, pain and scaling at week 16 and week 52 (CLEAR) were demonstrated in the Psoriasis Symptom Diary© in patients treated with secukinumab compared to patients treated with ustekinumab.
Statistically significant improvements (decreases) at week 12 from baseline in the scalp psoriasis study were demonstrated in patient reported signs and symptoms of scalp itching, pain and scaling compared to placebo.
Plaque psoriasis dose flexibility
A randomised, double-blind, multicentre study evaluated two maintenance dosing regimens (300 mg every 2 weeks [Q2W] and 300 mg every 4 weeks [Q4W]) administered by 150 mg pre-filled syringe in 331 patients weighing ≥90 kg with moderate to severe psoriasis. Patients were randomised 1:1 as follows:
- secukinumab 300 mg at weeks 0, 1, 2, 3, and 4 followed by the same dose every 2 weeks (Q2W) up to week 52 (n=165).
- secukinumab 300 mg at weeks 0, 1, 2, 3, and 4 followed by the same dose every 4 weeks (Q4W) up to week 16 (n=166).
- Patients randomised to receive secukinumab 300 mg Q4W who were PASI 90 responders at week 16 continued to receive the same dosing regimen up to week 52. Patients randomised to receive secukinumab 300 mg Q4W who were PASI 90 non-responders at week 16 either continued on the same dosing regimen, or were reassigned to receive secukinumab 300 mg Q2W up to week 52.
Overall, the efficacy response rates for the group treated with the every 2 weeks regimen were higher compared to the group treated with the every 4 weeks regimen (Table 7).
Table 7. Summary of clinical response in the plaque psoriasis dose flexibility study*:
| Week 16 | Week 52 | |||
|---|---|---|---|---|
| secukinumab 300 mg Q2W | secukinumab 300 mg Q4W | secukinumab 300 mg Q2W | secukinumab 300 mg Q4W1 | |
| Number of patients | 165 | 166 | 165 | 83 |
| PASI 90 response n (%) | 121 (73.2%)** | 92 (55.5%) | 126 (76.4%) | 44 (52.4%) |
| IGA mod 2011 "clear" or "almost clear" response n (%) | 122 (74.2%)2 | 109 (65.9%)2 | 125 (75.9%) | 46 (55.6%) |
* Multiple imputation
1 300 mg Q4W:patients continuously treated with 300 mg Q4W regardless of PASI 90 response status at week 16; 43 patients were PASI 90 responder at week 16 and 40 patients were PASI 90 non-responders at week 16
** One sided p-value = 0.0003 for primary endpoint of PASI 90 at week 16
2 Not statistically significant
In the PASI 90 non-responders at week 16 who were up-titrated to secukinumab 300 mg Q2W, the PASI 90 response rates improved compared to those who remained on the secukinumab 300 mg Q4W dosing regimen, while the IGA mod 2011 0/1 response rates remained stable over time in both treatment groups.
The safety profiles of the two dosing regimens, Cosentyx 300 mg administered every 4 weeks and Cosentyx 300 mg administered every 2 weeks, in patients weighing ≥90 kg were comparable and consistent with the safety profile reported in psoriasis patients.
Hidradenitis suppurativa
The safety and efficacy of secukinumab were assessed in 1 084 patients in two randomised, double-blind, placebo-controlled phase III studies in adult patients with moderate to severe hidradenitis suppurativa (HS) who were candidates for systemic biologic therapy. Patients were required to have at least five inflammatory lesions affecting at least two anatomical areas at baseline. In HS study 1 (SUNSHINE) and HS study 2 (SUNRISE), respectively, 4.6% and 2.8% of patients were Hurley stage I, 61.4% and 56.7% were Hurley stage II, and 34.0% and 40.5% were Hurley stage III.The proportion of patients weighing ≥90 kg was 54.7% in HS study 1 and 50.8% in HS study 2. Patients in these studies had a diagnosis of moderate to severe HS for a mean of 7.3 years and 56.3% of the study participants were female.
In HS study 1 and HS study 2, 23,8% and 23,2% of patients, respectively, were previously treated with a biologic. 82.3% and 83.6% of patients, respectively, were previously treated with systemic antibiotics.
HS study 1 evaluated 541 patients and HS study 2 evaluated 543 patients, of whom 12.8% and 10.7%, respectively, received concomitant stable-dose antibiotics. In both studies, patients randomised to secukinumab received 300 mg subcutaneously at weeks 0, 1, 2, 3 and 4, followed by 300 mg every 2 weeks (Q2W) or every 4 weeks (Q4W). At week 16, patients who were randomised to placebo were reassigned to receive secukinumab 300 mg at weeks 16, 17, 18, 19 and 20 followed by either secukinumab 300 mg Q2W or secukinumab 300 mg Q4W.
The primary endpoint in both studies (HS study 1 and HS study 2) was the proportion of patients achieving a Hidradenitis Suppurativa Clinical Response defined as at least a 50% decrease in abscesses and inflammatory nodules count with no increase in the number of abscesses and/or in the number of draining fistulae relative to baseline (HiSCR50) at week 16. Reduction in HS-related skin pain was assessed as a secondary endpoint on the pooled data of HS study 1 and HS study 2 using a Numerical Rating Scale (NRS) in patients who entered the studies with an initial baseline score of 3 or greater.
In HS study 1 and HS study 2, a higher proportion of patients treated with secukinumab 300 mg Q2W achieved a HiSCR50 response with a decrease in abscesses and inflammatory nodules (AN) count compared to placebo at week 16. In HS study 2, a difference in HiSCR50 response and AN count was also observed with the secukinumab 300 mg Q4W regimen. In the secukinumab 300 mg Q2W group in HS study 1 and in the secukinumab 300 mg Q4W group in HS study 2, a lower rate of patients experienced flares compared to placebo up to week 16. A higher proportion of patients treated with secukinumab 300 mg Q2W (pooled data) experienced a clinically relevant decrease in HS-related skin pain compared to placebo at week 16 (Table 8).
Table 8. Clinical response in HS study 1 and HS study 2 at week 161:
| HS study 1 | HS study 2 | |||||
| Placebo | 300 mg Q4W | 300 mg Q2W | Placebo | 300 mg Q4W | 300 mg Q2W | |
| Number of patients randomised | 180 | 180 | 181 | 183 | 180 | 180 |
| HiSCR50, n (%) | 61 (33.7) | 75 (41.8) | 82 (45.0*) | 57 (31.2) | 83 (46.1*) | 76 (42.3*) |
| AN count, mean % change from baseline | -24.3 | -42.4 | -46.8* | -22.4 | -45.5* | -39.3* |
| Flares, n (%) | 52 (29.0) | 42 (23.2) | 28 (15.4*) | 50 (27.0) | 28 (15.6*) | 36 (20.1) |
| Pooled data (HS study 1 and HS study 2) | ||||||
| Placebo | 300 mg Q4W | 300 mg Q2W | ||||
| <b.Number of patients with NRS ≥3 at baseline | 251 | 252 | 266 | |||
| ≥30% reduction in skin pain, NRS30 response, n (%) | 58 (23.0) | 84 (33.5) | 97 (36.6*) | |||
1 Multiple imputation was implemented to handle missing data
n: Rounded average number of subjects with responses in 100 imputations
* Statistically significant versus placebo based on the pre-defined hierarchy with overall alpha=0.05
AN: Abscesses and inflammatory Nodules; HiSCR: Hidradenitis Suppurativa Clinical Response; NRS: Numerical Rating Scale
In both studies, the onset of action of secukinumab occurred as early as week 2, the efficacy progressively increased to week 16 and was maintained up to week 52.
Improvements were seen for the primary and key secondary endpoints in HS patients regardless of previous or concomitant antibiotic treatment.
HiSCR50 responses were improved at week 16 in both biologic-naïve and biologic-exposed patients.
Greater improvements at week 16 from baseline compared to placebo were demonstrated in health-related quality of life as measured by the Dermatology Life Quality Index.
Psoriatic arthritis
The safety and efficacy of secukinumab were assessed in 1 999 patients in three randomised, double-blind, placebo-controlled phase III studies in patients with active psoriatic arthritis (≥3 swollen and ≥3 tender joints) despite non-steroidal anti-inflammatory drug (NSAID), corticosteroid or disease-modifying anti-rheumatic drug (DMARD) therapy. Patients with each subtype of PsA were enrolled in these studies, including polyarticular arthritis with no evidence of rheumatoid nodules, spondylitis with peripheral arthritis, asymmetric peripheral arthritis, distal interphalangeal involvement and arthritis mutilans. Patients in these studies had a diagnosis of PsA of at least five years. The majority of patients also had active psoriasis skin lesions or a documented history of psoriasis. Over 61% and 42% of the PsA patients had enthesitis and dactylitis at baseline, respectively. For all studies, the primary endpoint was American College of Rheumatology (ACR) 20 response. For Psoriatic Arthritis study 1 (PsA study 1) and Psoriatic Arthritis study 2 (PsA study 2), the primary endpoint was at week 24. For Psoriatic Arthritis study 3 (PsA study 3), the primary endpoint was at week 16 with the key secondary endpoint, the change from baseline in modified Total Sharp Score (mTSS), at week 24.
In PsA study 1, PsA study 2 and PsA study 3, 29%, 35% and 30% of patients, respectively, were previously treated with an anti-TNFα agent and discontinued the anti-TNFα agent for either lack of efficacy or intolerance (anti-TNFα-IR patients).
PsA study 1 (FUTURE 1) evaluated 606 patients, of whom 60.7% had concomitant MTX. Patients randomised to secukinumab received 10 mg/kg intravenously at weeks 0, 2, and 4, followed by either 75 mg or 150 mg subcutaneously every month starting at week 8. Patients randomised to placebo who were non-responders at week 16 (early rescue) and other placebo patients at week 24 were crossed over to receive secukinumab (either 75 mg or 150 mg subcutaneously) followed by the same dose every month.
PsA study 2 (FUTURE 2) evaluated 397 patients, of whom 46.6% had concomitant MTX. Patients randomised to secukinumab received 75 mg, 150 mg or 300 mg subcutaneously at weeks 0, 1, 2, 3 and 4, followed by the same dose every month. Patients randomised to receive placebo who were non-responders at week 16 (early rescue) were crossed over to receive secukinumab (either 150 mg or 300 mg subcutaneously) at week 16 followed by the same dose every month. Patients randomised to receive placebo who were responders at week 16 were crossed over to receive secukinumab (either 150 mg or 300 mg subcutaneously) at week 24 followed by the same dose every month.
PsA study 3 (FUTURE 5) evaluated 996 patients, of whom 50.1% had concomitant MTX. Patients were randomised to receive secukinumab 150 mg, 300 mg or placebo subcutaneously at weeks 0, 1, 2, 3 and 4, followed by the same dose every month, or a once monthly injection of secukinumab 150 mg (without loading). Patients randomised to receive placebo who were non-responders at week 16 (early rescue) were then crossed over to receive secukinumab (either 150 mg or 300 mg subcutaneously) at week 16 followed by the same dose every month. Patients randomised to receive placebo who were responders at week 16 were crossed over to receive secukinumab (either 150 mg or 300 mg subcutaneously) at week 24 followed by the same dose every month.
Signs and symptoms
Treatment with secukinumab resulted in significant improvement in measures of disease activity compared to placebo at weeks 16 and 24 (see Table 9).
Table 9. Clinical response in PsA study 2 and PsA study 3 at week 16 and week 24:
| PsA study 2 | PsA study 3 | |||||
|---|---|---|---|---|---|---|
| Placebo | 150 mg1 | 300 mg1 | Placebo | 150 mg1 | 300 mg1 | |
| Number of patients randomised | 98 | 100 | 100 | 332 | 220 | 222 |
| ACR20 response n (%) | ||||||
| Week 16 | 18 (18.4%) | 60 (60.0%***) | 57 (57.0%***) | 91◊ (27.4%) | 122◊ (55.5%***) | 139◊ (62.6%***) |
| Week 24 | 15◊ (15.3%) | 51◊ (51.0%***) | 54◊ (54.0%***) | 78 (23.5%) | 117 (53.2%***) | 141 (63.5%***) |
| ACR50 response n (%) | ||||||
| Week 16 | 6 (6.1%) | 37 (37.0%***) | 35 (35.0%***) | 27 (8.1%) | 79 (35.9%*) | 88 (39.6%*) |
| Week 24 | 7 (7.1%) | 35 (35.0%) | 35 (35.0%**) | 29 (8.7%) | 86 (39.1%***) | 97 (43.7%***) |
| ACR70 response n (%) | ||||||
| Week 16 | 2 (2.0%) | 17 (17.0%**) | 15 (15.0%**) | 14 (4.2%) | 40 (18.2%***) | 45 (20.3%***) |
| Week 24 | 1 (1.0%) | 21 (21.0%**) | 20 (20.0%**) | 13 (3.9%) | 53 (24.1%***) | 57 (25.7%***) |
| DAS28-CRP | ||||||
| Week 16 | -0.50 | -1.45*** | -1.51*** | -0.63 | -1.29* | -1.49* |
| Week 24 | -0.96 | -1.58** | -1.61** | -0.84 | -1.57*** | -1.68*** |
| Number of patients with ≥3% BSA psoriasis skin involvement at baseline | 43 (43.9%) | 58 (58.0%) | 41 (41.0%) | 162 (48.8%) | 125 (56.8%) | 110 (49.5%) |
| PASI 75 response n (%) | ||||||
| Week 16 | 3 (7.0%) | 33 (56.9%***) | 27 (65.9%***) | 20 (12.3%) | 75 (60.0%*) | 77 (70.0%*) |
| Week 24 | 7 (16.3%) | 28 (48.3%**) | 26 (63.4%***) | 29 (17.9%) | 80 (64.0%***) | 78 (70.9%***) |
| PASI 90 response n (%) | ||||||
| Week 16 | 3 (7.0%) | 22 (37.9%***) | 18 (43.9%***) | 15 (9.3%) | 46 (36.8%*) | 59 (53.6%*) |
| Week 24 | 4 (9.3%) | 19 (32.8%**) | 20 (48.8%***) | 19 (11.7%) | 51 (40.8%***) | 60 (54.5%***) |
| Dactylitis resolution n (%)† | ||||||
| Week 16 | 10 (37%) | 21 (65.6%*) | 26 (56.5%) | 40 (32.3%) | 46 (57.5%*) | 54 (65.9%*) |
| Week 24 | 4 (14.8%) | 16 (50.0%**) | 26 (56.5%**) | 42 (33.9%) | 51 (63.8%***) | 52 (63.4%***) |
| Enthesitis resolution n (%)‡ | ||||||
| Week 16 | 17 (26.2%) | 32 (50.0%**) | 32 (57.1%***) | 68 (35.4%) | 77 (54.6%*) | 78 (55.7%*) |
| Week 24 | 14 (21.5%) | 27 (42.2%*) | 27 (48.2%**) | 66 (34.4%) | 77 (54.6%***) | 86 (61.4%***) |
* p<0.05, *p<0.01, **p<0.001; versus placebo
All p-values are adjusted for multiplicity of testing based on pre-defined hierarchy at week 24 for PsA study 2, except for ACR70, Dactylitis and Enthesitis, which were exploratory endpoints and all endpoints at week 16.
All p-values are adjusted for multiplicity of testing based on pre-defined hierarchy at week 16 for PsA study 3, except for ACR70 which was an exploratory endpoint and all endpoints at week 24.
Non-responder imputation used for missing binary endpoint.
ACR: American College of Rheumatology; PASI: Psoriasis Area and Severity Index; DAS: Disease Activity Score; BSA: Body Surface Area
◊ Primary Endpoint
1 Secukinumab 150 mg or 300 mg s.c. at weeks 0, 1, 2, 3, and 4 followed by the same dose every month
† In patients with dactylitis at baseline (n=27, 32, 46, respectively for PsA study 2 and n=124, 80, 82, respectively for PsA study 3)
‡ In patients with enthesitis at baseline (n=65, 64, 56, respectively for PsA study 2 and n=192, 141, 140, respectively for PsA study 3)
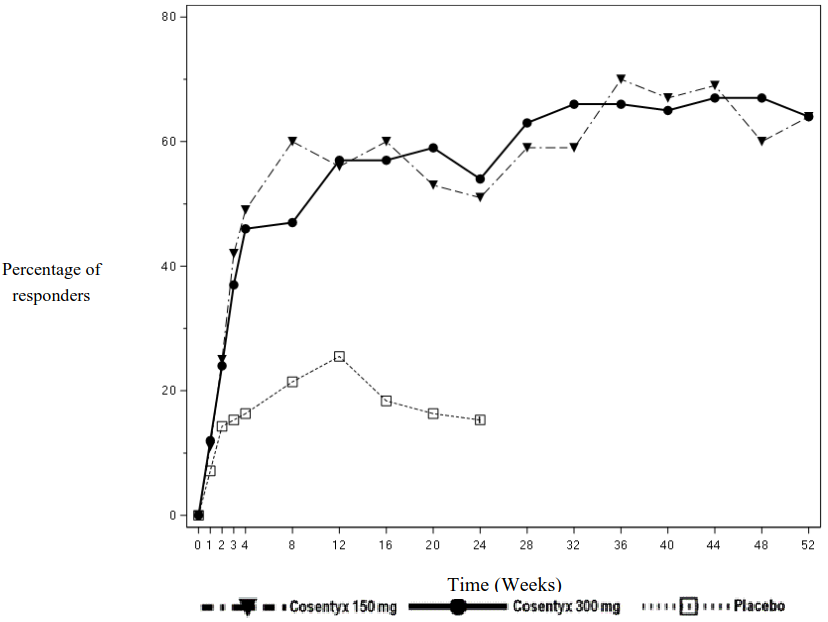
The onset of action of secukinumab occurred as early as week 2. Statistically significant difference in ACR 20 versus placebo was reached at week 3.
The percentage of patients achieving ACR 20 response by visit is shown in Figure 2.
Figure 2. ACR20 response in PsA study 2 over time up to week 52:
Similar responses for primary and key secondary endpoints were seen in PsA patients regardless of whether they were on concomitant MTX treatment or not. In PsA study 2, at week 24, secukinumab- treated patients with concomitant MTX use had a higher ACR 20 response (47.7% and 54.4% for 150 mg and 300 mg, respectively, compared to placebo 20.0%) and ACR 50 response (31.8% and 38.6% for 150 mg and 300 mg, respectively, compared to placebo 8.0%). Secukinumab-treated patients without concomitant MTX use had a higher ACR 20 response (53.6% and 53.6% for 150 mg and 300 mg, respectively, compared to placebo 10.4%) and ACR 50 response (37.5% and 32.1% for 150 mg and 300 mg, respectively, compared to placebo 6.3%).
In PsA study 2, both anti-TNFα-naive and anti-TNFα-IR secukinumab-treated patients had a significantly higher ACR 20 response compared to placebo at week 24, with a slightly higher response in the anti-TNFα-naive group (anti-TNFα-naive: 64% and 58% for 150 mg and 300 mg, respectively, compared to placebo 15.9%; anti-TNFα-IR: 30% and 46% for 150 mg and 300 mg, respectively, compared to placebo 14.3%). In the anti-TNFα-IR patients subgroup, only the 300 mg dose showed significantly higher response rate for ACR 20 compared to placebo (p<0.05) and demonstrated clinical meaningful benefit over 150 mg on multiple secondary endpoints. Improvements in the PASI 75 response were seen in both subgroups and the 300 mg dose showed statistically significant benefit in the anti-TNFα-IR patients.
Improvements were shown in all components of the ACR scores, including patient assessment of pain. In PsA study 2, the proportion of patients achieving a modified PsA Response Criteria (PsARC) response was greater in the secukinumab-treated patients (59.0% and 61.0% for 150 mg and 300 mg, respectively) compared to placebo (26.5%) at week 24.
In PsA study 1 and PsA study 2, efficacy was maintained up to week 104. In PsA study 2, among 200 patients initially randomised to secukinumab 150 mg and 300 mg, 178 (89%) patients were still on treatment at week 52. Of the 100 patients randomised to secukinumab 150 mg, 64, 39 and 20 had an ACR 20/50/70 response, respectively. Of the 100 patients randomised to secukinumab 300 mg, 64, 44 and 24 had an ACR 20/50/70 response, respectively.
Radiographic response
In PsA study 3, inhibition of progression of structural damage was assessed radiographically and expressed by the modified Total Sharp Score (mTSS) and its components, the Erosion Score (ES) and the Joint Space Narrowing Score (JSN). Radiographs of hands, wrists, and feet were obtained at baseline, week 16 and/or week 24 and scored independently by at least two readers who were blinded to treatment group and visit number. Secukinumab 150 mg and 300 mg treatment significantly inhibited the rate of progression of peripheral joint damage compared with placebo treatment as measured by change from baseline in mTSS at week 24 (Table 10).
Inhibition of progression of structural damage was also assessed in PsA study 1 at weeks 24 and 52, compared to baseline. Week 24 data are presented in Table 10.
Table 10. Change in modified Total Sharp Score in psoriatic arthritis:
| PsA study 3 | PsA study 1 | ||||
|---|---|---|---|---|---|
| Placebo n=296 | secukinumab 150 mg1 n=213 | secukinumab 300 mg1 n=217 | Placebo n=179 | secukinumab 150 mg2 n=185 | |
| Total score | |||||
| Baseline (SD) | 15.0 (38.2) | 13.5 (25.6) | 12.9 (23.8) | 28.4 (63.5) | 22.3 (48.0) |
| Mean change at week 24 | 0.50 | 0.13* | 0.02* | 0.57 | 0.13* |
* p<0.05 based on nominal, but non adjusted, p-value
1 secukinumab 150 mg or 300 mg s.c. at weeks 0, 1, 2, 3, and 4 followed by the same dose every month
2 10 mg/kg at weeks 0, 2 and 4 followed by subcutaneous doses of 75 mg or 150 mg
In PsA study 1, inhibition of structural damage was maintained with secukinumab treatment up to week 52.
In PsA study 3, the percentage of patients with no disease progression (defined as a change from baseline in mTSS of ≤0.5) from randomisation to week 24 was 80.3%, 88.5% and 73.6% for secukinumab 150 mg, 300 mg and placebo, respectively. An effect of inhibition of structural damage was observed in anti-TNFα-naïve and anti-TNFα-IR patients and in patients treated with and without concomitant MTX.
In PsA study 1, the percentage of patients with no disease progression (defined as a change from baseline in mTSS of ≤0.5) from randomisation to week 24 was 82.3% in secukinumab 10 mg/kg intravenous load – 150 mg subcutaneous maintenance and 75.7% in placebo. The percentage of patients with no disease progression from week 24 to week 52 for secukinumab 10 mg/kg intravenous load – followed by 150 mg subcutaneous maintenance and for placebo patients who switched to 75 mg or 150 mg subcutaneous every 4 weeks at week 16 or week 24 was 85.7% and 86.8%, respectively.
Axial manifestations in PsA
A randomised, double-blind, placebo-controlled study (MAXIMISE) assessed the efficacy of secukinumab in 485 PsA patients with axial manifestations who were naive to biologic treatment and responded inadequately to NSAIDs. The primary variable of at least a 20% improvement in Assessment of SpondyloArthritis International Society (ASAS 20) criteria at week 12 was met. Treatment with secukinumab 300 mg and 150 mg compared to placebo also resulted in greater improvement in signs and symptoms (including decreases from baseline in spinal pain) and improvement in physical function (see Table 11).
Table 11. Clinical response on MAXIMISE study at week 12:
| Placebo (n=164) | 150 mg (n=157) | 300 mg (n=164) | |
|---|---|---|---|
| ASAS 20 response, % (95% CI) | 31.2 (24.6, 38.7) | 66.3 (58.4, 73.3)* | 62.9 (55.2, 70.0)* |
| ASAS 40 response, % (95% CI) | 12.2 (7.8, 18.4) | 39.5 (32.1, 47.4)** | 43.6 (36.2, 51.3)** |
| BASDAI 50, % (95% CI) | 9.8 (5.9, 15.6) | 32.7 (25.8, 40.5)** | 37.4 (30.1, 45.4)** |
| Spinal pain, VAS (95% CI) | -13.6 (-17.2, -10.0) | -28.5 (-32.2, -24.8)** | -26.5 (-30.1, -22.9)** |
| Physical function, HAQ-DI (95% CI) | -0.155 (-0.224, -0.086) | -0.330 (-0.401, -0.259)** | -0.389 (-0.458, -0.320)** |
* p<0.0001; versus placebo using multiple imputation.
** Comparison versus placebo was not adjusted for multiplicity.
ASAS: Assessment of SpondyloArthritis International Society Criteria; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; VAS: Visual Analog Scale; HAQ-DI: Health Assessment Questionnaire – Disability Index.
Improvement in ASAS 20 and ASAS 40 for both secukinumab doses were observed by week 4 and were maintained up to 52 weeks.
Physical function and health-related quality of life
In PsA study 2 and PsA study 3, patients treated with secukinumab 150 mg (p=0.0555 and p<0.0001) and 300 mg (p=0.0040 and p<0.0001) showed improvement in physical function compared to patients treated with placebo as assessed by Health Assessment Questionnaire-Disability Index (HAQ-DI) at week 24 and week 16, respectively. Improvements in HAQ-DI scores were seen regardless of previous anti-TNFα exposure. Similar responses were seen in PsA study 1.
Secukinumab-treated patients reported significant improvements in health-related quality of life as measured by the Short Form-36 Health Survey Physical Component Summary (SF-36 PCS) score (p<0.001). There were also statistically significant improvements demonstrated in exploratory endpoints assessed by the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scores for 150 mg and 300 mg compared to placebo (7.97, 5.97 versus 1.63, respectively) and these improvements were maintained up to week 104 in PsA study 2.
Similar responses were seen in PsA study 1 and efficacy was maintained up to week 52.
Axial spondyloarthritis (axSpA)
Ankylosing spondylitis (AS) / Radiographic axial spondyloarthritis
The safety and efficacy of secukinumab were assessed in 816 patients in three randomised, double-blind, placebo-controlled phase III studies in patients with active ankylosing spondylitis (AS) with a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥4 despite non-steroidal anti-inflammatory drug (NSAID), corticosteroid or disease-modifying anti-rheumatic drug (DMARD) therapy. Patients in Ankylosing Spondylitis study 1 (AS study 1) and Ankylosing Spondylitis study 2 (AS study 2) had a diagnosis of AS for a median of 2.7 to 5.8 years. For both studies, the primary endpoint was at least a 20% improvement in Assessment of SpondyloArthritis International Society (ASAS 20) criteria at week 16.
In Ankylosing Spondylitis study 1 (AS study 1), Ankylosing Spondylitis study 2 (AS study 2), and Ankylosing Spondylitis study 3 (AS study 3), 27.0%, 38.8%, and 23.5% of patients, respectively, were previously treated with an anti-TNFα agent and discontinued the anti-TNFα agent for either lack of efficacy or intolerance (anti-TNFα-IR patients).
AS study 1 (MEASURE 1) evaluated 371 patients, of whom 14.8% and 33.4% used concomitant MTX or sulfasalazine, respectively. Patients randomised to secukinumab received 10 mg/kg intravenously at weeks 0, 2, and 4, followed by either 75 mg or 150 mg subcutaneously every month starting at week 8. Patients randomised to placebo who were non-responders at week 16 (early rescue) and all other placebo patients at week 24 were crossed over to receive secukinumab (either 75 mg or 150 mg subcutaneously), followed by the same dose every month.
AS study 2 (MEASURE 2) evaluated 219 patients, of whom 11.9% and 14.2% used concomitant MTX or sulfasalazine, respectively. Patients randomised to secukinumab received 75 mg or 150 mg subcutaneously at weeks 0, 1, 2, 3 and 4, followed by the same dose every month. At week 16, patients who were randomised to placebo at baseline were re-randomised to receive secukinumab (either 75 mg or 150 mg subcutaneously) every month.
AS study 3 (MEASURE 3) evaluated 226 patients, of whom 13.3% and 23.5% used concomitant MTX or sulfasalazine, respectively. Patients randomised to secukinumab received 10 mg/kg intravenously at weeks 0, 2, and 4, followed by either 150 mg or 300 mg subcutaneously every month. At week 16, patients who were randomised to placebo at baseline were re-randomised to receive secukinumab (either 150 mg or 300 mg subcutaneously) every month. The primary endpoint was ASAS 20 at week 16. Patients were blinded to the treatment regimen up to week 52, and the study continued to week 156.
Signs and symptoms:
In AS study 2, treatment with secukinumab 150 mg resulted in greater improvement in measures of disease activity compared with placebo at week 16 (see Table 12).
Table 12. Clinical response in AS study 2 at week 16:
| Outcome (p-value versus placebo) | Placebo (n=74) | 75 mg (n=73) | 150 mg (n=72) |
|---|---|---|---|
| ASAS 20 response, % | 28.4 | 41.1 | 61.1*** |
| ASAS 40 response, % | 10.8 | 26.0 | 36.1*** |
| hsCRP, (post-BSL/BSL ratio) | 1.13 | 0.61 | 0.55*** |
| ASAS 5/6, % | 8.1 | 34.2 | 43.1*** |
| ASAS partial remission, % | 4.1 | 15.1 | 13.9 |
| BASDAI 50, % | 10.8 | 24.7* | 30.6** |
| ASDAS-CRP major improvement | 4.1 | 15.1* | 25.0*** |
* p<0.05, *p<0.01, **p<0.001; versus placebo
All p-values adjusted for multiplicity of testing based on pre-defined hierarchy, except BASDAI 50 and ASDAS-CRP
Non-responder imputation used for missing binary endpoint
ASAS: Assessment of SpondyloArthritis International Society Criteria; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; hsCRP: high-sensitivity C-reactive protein; ASDAS: Ankylosing Spondylitis Disease Activity Score; BSL: baseline
The onset of action of secukinumab 150 mg occurred as early as week 1 for ASAS 20 and week 2 for ASAS 40 (superior to placebo) in AS study 2.
ASAS 20 responses were improved at week 16 in both anti-TNFα-naïve patients (68.2% versus 31.1%; p<0.05) and anti-TNFα-IR patients (50.0% versus 24.1%; p<0.05) for secukinumab 150 mg compared with placebo, respectively.
In AS study 1 and AS study 2, secukinumab-treated patients (150 mg in AS study 2 and both regimens in AS study 1) demonstrated significantly improved signs and symptoms at week 16, with comparable magnitude of response and efficacy maintained up to week 52 in both anti-TNFα-naive and anti-TNFα-IR patients. In AS study 2, among 72 patients initially randomised to secukinumab 150 mg, 61 (84.7%) patients were still on treatment at week 52. Of the 72 patients randomised to secukinumab 150 mg, 45 and 35 had an ASAS 20/40 response, respectively.
In AS study 3, patients treated with secukinumab (150 mg and 300 mg) demonstrated improved signs and symptoms, and had comparable efficacy responses regardless of dose that were superior to placebo at week 16 for the primary endpoint (ASAS 20). Overall, the efficacy response rates for the 300 mg group were consistently greater compared to the 150 mg group for the secondary endpoints. During the blinded period, the ASAS 20 and ASAS 40 responses were 69.7% and 47.6% for 150 mg and 74.3% and 57.4% for 300 mg at week 52, respectively. The ASAS 20 and ASAS 40 responses were maintained up to week 156 (69.5% and 47.6% for 150 mg versus 74.8% and 55.6% for 300 mg). Greater response rates favouring 300 mg were also observed for ASAS partial remission (ASAS PR) response at week 16 and were maintained up to week 156. Larger differences in response rates, favouring 300 mg over 150 mg, were observed in anti-TNFα-IR patients (n=36) compared to anti-TNFα-naïve patients (n=114).
Spinal mobility:
Patients treated with secukinumab 150 mg showed improvements in spinal mobility as measured by change from baseline in BASMI at week 16 for both AS study 1 (-0.40 versus -0.12 for placebo; p=0.0114) and AS study 2 (-0.51 versus -0.22 for placebo; p=0.0533). These improvements were sustained up to week 52.
Physical function and health-related quality of life:
In AS study 1 and study 2, patients treated with secukinumab 150 mg showed improvements in health-related quality of life as measured by AS Quality of Life Questionnaire (ASQoL) (p=0.001) and SF-36 Physical Component Summary (SF-36PCS) (p<0.001). Patients treated with secukinumab 150 mg also showed statistically significant improvements on exploratory endpoints in physical function as assessed by the Bath Ankylosing Spondylitis Functional Index (BASFI) compared to placebo (2.15 versus -0.68), and in fatigue as assessed by the Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-Fatigue) scale compared to placebo (8.10 versus 3.30). These improvements were sustained up to week 52.
Non-radiographic axial spondyloarthritis (nr-axSpA)
The safety and efficacy of secukinumab were assessed in 555 patients in one randomised, double-blind, placebo-controlled phase III study (PREVENT), consisting of a 2-year core phase and a 2-year extension phase, in patients with active non-radiographic axial spondyloarthritis (nr-axSpA) fulfilling the Assessment of SpondyloArthritis International Society (ASAS) classification criteria for axial spondyloarthritis (axSpA) with no radiographic evidence of changes in the sacroiliac joints that would meet the modified New York criteria for ankylosing spondylitis (AS). Patients enrolled had active disease, defined as a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥4, a Visual Analogue Scale (VAS) for total back pain of ≥40 (on a scale of 0-100 mm), despite current or previous non-steroidal anti-inflammatory drug (NSAID) therapy and increased C-reactive protein (CRP) and/or evidence of sacroiliitis on Magnetic Resonance Imaging (MRI). Patients in this study had a diagnosis of axSpA for a mean of 2.1 to 3.0 years and 54% of the study participants were female.
In the PREVENT study, 9.7% of patients were previously treated with an anti-TNFα agent and discontinued the anti-TNFα agent for either lack of efficacy or intolerance (anti-TNFα-IR patients). In the PREVENT study, 9.9% and 14.8% of patients used concomitant MTX or sulfasalazine, respectively. In the double-blind period, patients received either placebo or secukinumab for 52 weeks. Patients randomised to secukinumab received 150 mg subcutaneously at weeks 0, 1, 2, 3 and 4 followed by the same dose every month, or a once monthly injection of secukinumab 150 mg. The primary endpoint was at least 40% improvement in Assessment of SpondyloArthritis International Society (ASAS 40) at Week 16 in anti-TNFα-naive patients.
Signs and symptoms:
In the PREVENT study, treatment with secukinumab 150 mg resulted in significant improvements in the measures of disease activity compared to placebo at week 16. These measures include ASAS 40, ASAS 5/6, BASDAI score, BASDAI 50, high-sensitivity CRP (hsCRP), ASAS 20 and ASAS partial remission response compared to placebo (Table 13). Responses were maintained up to week 52.
Table 13. Clinical response in the PREVENT study at week 16:
| Outcome (p-value versus placebo) | Placebo | 150 mg1 |
|---|---|---|
| Number of anti-TNFα-naive patients randomised | 171 | 164 |
| ASAS 40 response, % | 29.2 | 41.5* |
| Total number of patients randomised | 186 | 185 |
| ASAS 40 response, % | 28.0 | 40.0* |
| ASAS 5/6, % | 23.7 | 40.0* |
| BASDAI, LS mean change from baseline score | -1.46 | -2.35* |
| BASDAI 50, % | 21.0 | 37.3* |
| hsCRP, (post-BSL/BSL ratio) | 0.91 | 0.64* |
| ASAS 20 response, % | 45.7 | 56.8* |
| ASAS partial remission, % | 7.0 | 21.6* |
* p<0.05 versus placebo
All p-values adjusted for multiplicity of testing based on pre-defined hierarchy
Non-responder imputation used for missing binary endpoint
1 secukinumab 150 mg s.c. at weeks 0, 1, 2, 3, and 4 followed by the same dose every month
ASAS: Assessment of SpondyloArthritis International Society Criteria; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; hsCRP: high-sensitivity C-reactive protein; BSL: baseline; LS: Least square
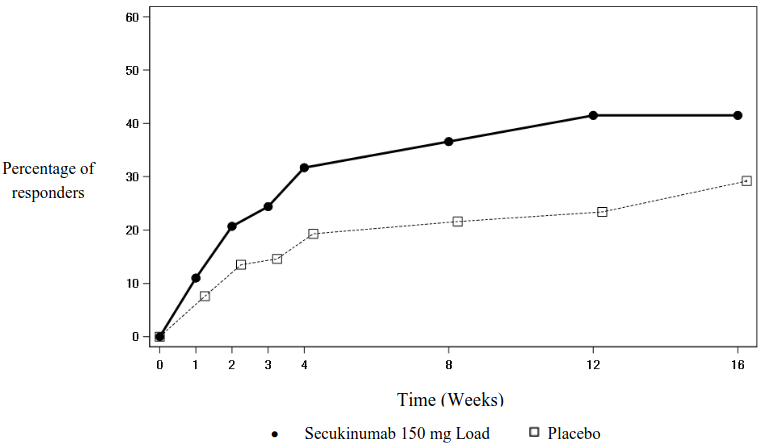
The onset of action of secukinumab 150 mg occurred as early as week 3 for ASAS 40 in anti-TNFα naive patients (superior to placebo) in the PREVENT study. The percentage of patients achieving an ASAS 40 response in anti-TNFα naive patients by visit is shown in Figure 3.
Figure 3. ASAS 40 responses in anti-TNFα naive patients in the PREVENT study over time up to week 16:
ASAS 40 responses were also improved at week 16 in anti-TNFα-IR patients for secukinumab 150 mg compared with placebo.
Physical function and health-related quality of life:
Patients treated with secukinumab 150 mg showed statistically significant improvements by week 16 compared to placebo-treated patients in physical function as assessed by the BASFI (week 16: -1.75 versus -1.01, p<0.05). Patients treated with secukinumab reported significant improvements compared to placebo-treated patients by week 16 in health-related quality of life as measured by ASQoL (LS mean change: week 16: -3.45 versus -1.84, p<0.05) and SF-36 Physical Component Summary (SF-36 PCS) (LS mean change: week 16: 5.71 versus 2.93, p<0.05). These improvements were sustained up to week 52.
Spinal mobility:
Spinal mobility was assessed by BASMI up to week 16. Numerically greater improvements were demonstrated in patients treated with secukinumab compared with placebo-treated patients at weeks 4, 8, 12 and 16.
Inhibition of inflammation in magnetic resonance imaging (MRI):
Signs of inflammation were assessed by MRI at baseline and week 16 and expressed as change from baseline in Berlin SI-joint oedema score for sacroiliac joints and ASspiMRI-a score and Berlin spine score for the spine. Inhibition of inflammatory signs in both sacroiliac joints and the spine was observed in patients treated with secukinumab. Mean change from baseline in Berlin SI-joint oedema score was -1.68 for patients treated with secukinumab 150 mg (n=180) versus -0.39 for the placebo-treated patients (n=174) (p<0.05).
Paediatric population
Paediatric plaque psoriasis
Secukinumab has been shown to improve signs and symptoms, and health-related quality of life in paediatric patients 6 years and older with plaque psoriasis (see Tables 15 and 17).
Severe plaque psoriasis
The safety and efficacy of secukinumab were assessed in a randomised, double-blind, placebo and etanercept-controlled phase III study in paediatric patients from 6 to <18 years of age with severe plaque psoriasis, as defined by a PASI score ≥20, an IGA mod 2011 score of 4, and BSA involvement of ≥10%, who were candidates for systemic therapy. Approximately 43% of the patients had prior exposure to phototherapy, 53% to conventional systemic therapy, 3% to biologics, and 9% had concomitant psoriatic arthritis.
The paediatric psoriasis study 1 evaluated 162 patients who were randomised to receive low dose secukinumab (75 mg for body weight <50 kg or 150 mg for body weight ≥50 kg), high dose secukinumab (75 mg for body weight <25 kg, 150 mg for body weight between ≥25 kg and <50 kg, or 300 mg for body weight ≥50 kg), or placebo at weeks 0, 1, 2, 3, and 4 followed by the same dose every 4 weeks, or etanercept. Patients randomised to etanercept received 0.8 mg/kg weekly (up to a maximum of 50 mg). Patient distribution by weight and age at randomisation is described in Table 14.
Table 14. Patient distribution by weight and age for paediatric psoriasis study 1:
| Randomisation strata | Description | Secukinumab low dose n=40 | Secukinumab high dose n=40 | Placebo n=41 | Etanercept n=41 | Total N=162 |
|---|---|---|---|---|---|---|
| Age | 6 - <12 years | 8 | 9 | 10 | 10 | 37 |
| ≥12 - <18 years | 32 | 31 | 31 | 31 | 125 | |
| Weight | <25 kg | 2 | 3 | 3 | 4 | 12 |
| ≥25-<50 kg | 17 | 15 | 17 | 16 | 65 | |
| ≥50 kg | 21 | 22 | 21 | 21 | 85 |
Patients randomised to receive placebo who were non-responders at week 12 were switched to either the secukinumab low or high dose group (dose based on body weight group) and received study drug at weeks 12, 13, 14, and 15, followed by the same dose every 4 weeks starting at week 16. The co-primary endpoints were the proportion of patients who achieved a PASI 75 response and IGA mod 2011 'clear' or 'almost clear' (0 or 1) response at week 12.
During the 12 week placebo-controlled period, the efficacy of both the low and the high dose of secukinumab was comparable for the co-primary endpoints. The odds ratio estimates in favour of both secukinumab doses were statistically significant for both the PASI 75 and IGA mod 2011 0 or 1 responses.
All patients were followed for efficacy and safety during the 52 weeks following the first dose. The proportion of patients achieving PASI 75 and IGA mod 2011 'clear' or 'almost clear' (0 or 1) responses showed separation between secukinumab treatment groups and placebo at the first post-baseline visit at week 4, the difference becoming more prominent at week 12. The response was maintained throughout the 52 week time period (see Table 15). Improvement in PASI 50, 90, 100 responder rates and Children's Dermatology Life Quality Index (CDLQI) scores of 0 or 1 were also maintained throughout the 52 week time period.
In addition, PASI 75, IGA 0 or 1, PASI 90 response rates at weeks 12 and 52 for both secukinumab low and high dose groups were higher than the rates for patients treated with etanercept (see Table 15). Beyond week 12, efficacy of both the low and the high dose of secukinumab was comparable although the efficacy of the high dose was higher for patients ≥50 kg. The safety profiles of the low dose and the high dose were comparable and consistent with the safety profile in adults.
Table 15. Summary of clinical response in severe paediatric psoriasis at weeks 12 and 52 (paediatric psoriasis study 1)*:
| Response criterion | Treatment comparison 'test' vs. 'control' | 'test' n**/m (%) | 'control' n**/m (%) | odds ratio estimate (95% CI) | p-value |
|---|---|---|---|---|---|
| At week 12*** | |||||
| PASI 75 | secukinumab low dose vs. placebo | 32/40 (80.0) | 6/41 (14.6) | 25.78 (7.08, 114.66) | <0.0001 |
| secukinumab high dose vs. placebo | 31/40 (77.5) | 6/41 (14.6) | 22.65 (6.31, 98.93) | <0.0001 | |
| secukinumab low dose vs. etanercept | 32/40 (80.0) | 26/41 (63.4) | 2.25 (0.73, 7.38) | ||
secukinumab high dose vs. etanercept |<>31/40 (77.5) |<>26/41 (63.4) |<>1.92 (0.64, 6.07) | |
|/4< IGA 0/1 |secukinumab low dose vs. placebo |<>28/40 (70.0) |<>2/41 (4.9) 5|<>1.77 (10.02, 538.64) |<> <0.0001|
|secukinumab high dose vs. placebo |<>24/40 (60.0) |<>2/41 (4.9) |<>32.52 (6.48, 329.52) |<> <0.0001|
|secukinumab low dose vs. etanercept |<>28/40 (70.0) |<>14/41 (34.1) |<>4.49 (1.60, 13.42) | |
|secukinumab high dose vs. etanercept |<>24/40 (60.0) |<>14/41 (34.1) |<>2.86 (1.05, 8.13) | |
|/4< PASI 90 |secukinumab low dose vs. placebo |<>29/40 (72.5) |<>1/41 (2.4) 1|<>33.67 (16.83, 6395.22) |<> <0.0001|
|secukinumab high dose vs. placebo |<>27/40 (67.5) |<>1/41 (2.4) |<>102.86 (13.22, 4850.13) |<> <0.0001|
|secukinumab low dose vs. etanercept |<>29/40 (72.5) |<>12/41 (29.3) |<>7.03 (2.34, 23.19) | |
|secukinumab high dose vs. etanercept |<>27/40 (67.5) |<>12/41 (29.3) |<>5.32 (1.82, 16.75) | |
|\6<> At week 52 |
|/2< PASI 75 |secukinumab low dose vs. etanercept |<>35/40 (87.5) |<>28/41 (68.3) |<>3.12 (0.91, 12.52) |
|secukinumab high dose vs. etanercept |<>35/40 (87.5) |<>28/41 (68.3) |<>3.09 (0.90, 12.39) | |
|/2< IGA 0/1 |secukinumab low dose vs. etanercept |<>29/40 (72.5) |<>23/41 (56.1) |<>2.02 (0.73, 5.77) | |
|secukinumab high dose vs. etanercept |<>30/40 (75.0) |<>23/41 (56.1) |<>2.26 (0.81, 6.62) | |
|/2< PASI 90 |secukinumab low dose vs. etanercept |<>30/40 (75.0) |<>21/41 (51.2) |<>2.85 (1.02, 8.38) | |
|secukinumab high dose vs. etanercept |<>32/40 (80.0) |<>21/41 (51.2) |<>3.69 (1.27, 11.61) | |
* non-responder imputation was used to handle missing values
** n is the number of responders, m = number of patients evaluable
*** extended visit window at week 12
Odds ratio, 95% confidence interval, and p-value are from an exact logistic regression model with treatment group, baseline body-weight category and age category as factors
A higher proportion of paediatric patients treated with secukinumab reported improvement in health-related quality of life as measured by a CDLQI score of 0 or 1 compared to placebo at week 12 (low dose 44.7%, high dose 50%, placebo 15%). Over time up to and including week 52 both secukinumab dose groups were numerically higher than the etanercept group (low dose 60.6%, high dose 66.7%, etanercept 44.4%).
Moderate to severe plaque psoriasis
Secukinumab was predicted to be effective for the treatment of paediatric patients with moderate plaque psoriasis based on the demonstrated efficacy and exposure response relationship in adult patients with moderate to severe plaque psoriasis, and the similarity of the disease course, pathophysiology, and drug effect in adult and paediatric patients at the same exposure levels.
Moreover, the safety and efficacy of secukinumab was assessed in an open-label, two-arm, parallel-group, multicentre phase III study in paediatric patients from 6 to <18 years of age with moderate to severe plaque psoriasis, as defined by a PASI score ≥12, an IGA mod 2011 score of ≥3, and BSA involvement of ≥10%, who were candidates for systemic therapy.
The paediatric psoriasis study 2 evaluated 84 patients who were randomised to receive low dose secukinumab (75 mg for body weight <50 kg or 150 mg for body weight ≥50 kg) or high dose secukinumab (75 mg for body weight <25 kg, 150 mg for body weight between ≥25 kg and <50 kg, or 300 mg for body weight ≥50 kg) at weeks 0, 1, 2, 3, and 4 followed by the same dose every 4 weeks. Patient distribution by weight and age at randomisation is described in Table 16.
Table 16. Patient distribution by weight and age for paediatric psoriasis study 2:
| Sub-groups | Description | Secukinumab low dose n=42 | Secukinumab high dose n=42 | Total N=84 |
|---|---|---|---|---|
| Age | 6 - <12 years | 17 | 16 | 33 |
| ≥12 - <18 years | 25 | 26 | 51 | |
| Weight | <25 kg | 4 | 4 | 8 |
| ≥25 - <50 kg | 13 | 12 | 25 | |
| ≥50 kg | 25 | 26 | 51 |
The co-primary endpoints were the proportion of patients who achieved a PASI 75 response and IGA mod 2011 'clear' or 'almost clear' (0 or 1) response at week 12.
The efficacy of both the low and the high dose of secukinumab was comparable and showed statistically significant improvement compared to historical placebo for the co-primary endpoints. The estimated posterior probability of a positive treatment effect was 100%.
Patients were followed for efficacy over a 52 week period after first administration. Efficacy (defined as PASI 75 response and IGA mod 2011 'clear' or 'almost clear' [0 or 1]) was observed as early as the first post-baseline visit at week 2, and the proportion of patients who achieved a PASI 75 response and IGA mod 2011 'clear' or 'almost clear' (0 or 1) increased up to week 24 and were sustained until week 52. Improvement in PASI 90 and PASI 100 were also observed at week 12, increased up to week 24, and were sustained until week 52 (see Table 17).
The safety profiles of the low dose and the high dose were comparable and consistent with the safety profile in adults.
Table 17. Summary of clinical response in moderate to severe paediatric psoriasis at weeks 12 and 52 (paediatric psoriasis study 2)*:
| Week 12 | Week 52 | |||
|---|---|---|---|---|
| Secukinumab low dose | Secukinumab high dose | Secukinumab low dose | Secukinumab high dose | |
| Number of patients | 42 | 42 | 42 | 42 |
| PASI 75 response n (%) | 39 (92.9%) | 39 (92.9%) | 37 (88.1%) | 38 (90.5%) |
| IGA mod 2011 'clear' or 'almost clear' response n (%) | 33 (78.6%) | 35 (83.3%) | 36 (85.7%) | 35 (83.3%) |
| PASI 90 response n (%) | 29 (69%) | 32 (76.2%) | 32 (76.2%) | 35 (83.3%) |
| PASI 100 response n (%) | 25 (59.5%) | 23 (54.8%) | 22 (52.4%) | 29 (69.0%) |
* non-responder imputation was used to handle missing values
These outcomes in the paediatric moderate to severe plaque psoriasis population confirmed the predictive assumptions based on the efficacy and exposure response relationship in adult patients, mentioned above.
In the low dose group, 50% and 70.7% of patients achieved a CDLQI 0 or 1 score at weeks 12 and 52, respectively. In the high dose group, 61.9% and 70.3% achieved a CDLQI 0 or 1 score at weeks 12 and 52, respectively.
Juvenile idiopathic arthritis (JIA)
Enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA)
The efficacy and safety of secukinumab were assessed in 86 patients in a 3-part, double-blind, placebo-controlled, event-driven, randomised, phase III study in patients 2 to <18 years of age with active ERA or JPsA as diagnosed based on a modified International League of Associations for Rheumatology (ILAR) JIA classification criteria. The study consisted of an open-label portion (Part 1) where all patients received secukinumab until week 12. Patients demonstrating a JIA ACR 30 response at week 12 entered into the Part 2 double-blind phase and were randomised 1:1 to continue treatment with secukinumab or to begin treatment with placebo (randomised withdrawal) until week 104 or until a flare occured. Patients who flared then entered open-label secukinumab treatment until week 104 (Part 3).
The JIA patient subtypes at study entry were: 60.5% ERA and 39.5% JPsA, who either had inadequate response or were intolerant to ≥1 disease-modifying antirheumatic drugs (DMARDs) and ≥1 non- steroidal anti-inflammatory drugs (NSAIDs). At baseline, MTX use was reported for 65.1% of patients; (63.5% [33/52] of ERA patients and 67.6% [23/34] of JPsA patients). There were 12 out of 52 ERA patients concomitantly treated with sulfasalazine (23.1%). Patients with a body weight at baseline <50 kg (n=30) were given a dose of 75 mg and patients with a body weight ≥50 kg (n=56) were given a dose of 150 mg. Age at baseline ranged from 2 to 17 years, with 3 patients between 2 to <6 years, 22 patients 6 to <12 years and 61 patients 12 to <18 years. At baseline the Juvenile Arthritis Disease Activity Score (JADAS)-27 was 15.1 (SD:7.1).
The primary endpoint was time to flare in the randomised withdrawal period (Part 2). Disease flare was defined as a ≥30% worsening in at least three of the six JIA ACR response criteria and ≥30% improvement in not more than one of the six JIA ACR response criteria and a minimum of two active joints.
At the end of Part 1, 75 out of 86 (87.2%) patients demonstrated a JIA ACR 30 response and entered into Part 2.
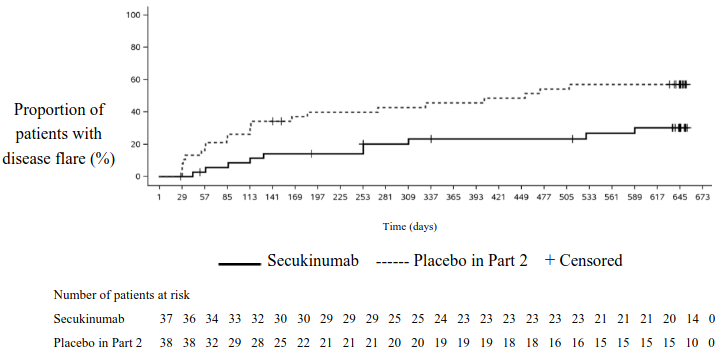
The study met its primary endpoint by demonstrating a statistically significant prolongation in the time to disease flare in patients treated with secukinumab compared to placebo in Part 2. The risk of flare was reduced by 72% for patients on secukinumab compared with patients on placebo in Part 2 (Hazard ratio=0.28, 95% CI: 0.13 to 0.63, p<0.001) (Figure 4 and Table 18). During Part 2, a total of 21 patients in the placebo group experienced a flare event (11 JPsA and 10 ERA) compared with 10 patients in the secukinumab group (4 JPsA and 6 ERA).
Figure 4. Kaplan-Meier estimates of the time to disease flare in Part 2:
Table 18. Survival analysis of time to disease flare – Part 2:
| Secukinumab (N=37) | Placebo in Part 2 (N=38) | |
|---|---|---|
| Number of flare events at the end of Part 2, n (%) | 10 (27.0) | 21 (55.3) |
| Kaplan-Meier estimates: | ||
| Median, in days (95% CI) | NC (NC, NC) | 453.0 (114.0, NC) |
| Flare-free rate at 6 months (95% CI) | 85.8 (69.2, 93.8) | 60.1 (42.7, 73.7) |
| Flare-free rate at 12 months (95% CI) | 76.7 (58.7, 87.6) | 54.3 (37.1, 68.7) |
| Flare-free rate at 18 months (95% CI) | 73.2 (54.6, 85.1) | 42.9 (26.7, 58.1) |
| Hazard ratio to placebo: Estimate (95% CI) | 0.28 (0.13, 0.63) | |
| Stratified log-rank test p-value | <0.001** | |
Analysis was conducted on all randomised patients who received at least one dose of study drug in Part 2.
Secukinumab: all patients who did not take any placebo. Placebo in Part 2: all patients who took placebo in Part 2 and secukinumab in other period/s. NC = Not calculable. ** = Statistically significant on one-sided significance level 0.025.
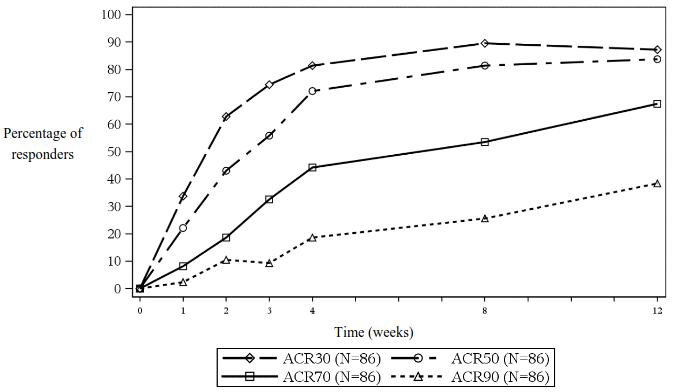
In open-label Part 1, all patients received secukinumab until week 12. At week 12, 83.7%, 67.4%, and 38.4% of children were JIA ACR 50, 70 and 90 responders, respectively (Figure 5). The onset of action of secukinumab occurred as early as week 1. At week 12 the JADAS-27 score was 4.64 (SD:4.73) and the mean decrease from baseline in JADAS-27 was -10.487 (SD:7.23).
Figure 5. JIA ACR 30/50/70/90 response for subjects up to week 12 in Part 1*:
* non-responder imputation was used to handle missing values
The data in the 2 to <6 age group were inconclusive due to the low number of patients below 6 years of age enrolled in the study.
The European Medicines Agency has waived the obligation to submit the results of studies with Cosentyx in plaque psoriasis in paediatric patients aged from birth to less than 6 years and in chronic idiopathic arthritis for paediatric patients aged from birth to less than 2 years (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Most pharmacokinetics properties observed in patients with plaque psoriasis, psoriatic arthritis and ankylosing spondylitis were similar.
Absorption
Following a single subcutaneous dose of 300 mg as a liquid formulation in healthy volunteers, secukinumab reached peak serum concentrations of 43.2±10.4 μg/ml between 2 and 14 days post dose.
Based on population pharmacokinetic analysis, following a single subcutaneous dose of either 150 mg or 300 mg in plaque psoriasis patients, secukinumab reached peak serum concentrations of 13.7±4.8 μg/ml or 27.3±9.5 μg/ml, respectively, between 5 and 6 days post dose.
After initial weekly dosing during the first month, time to reach the maximum concentration was between 31 and 34 days based on population pharmacokinetic analysis.
On the basis of simulated data, peak concentrations at steady-state (Cmax,ss) following subcutaneous administration of 150 mg or 300 mg were 27.6 μg/ml and 55.2 μg/ml, respectively. Population pharmacokinetic analysis suggests that steady-state is reached after 20 weeks with monthly dosing regimens.
Compared with exposure after a single dose, the population pharmacokinetic analysis showed that patients exhibited a 2-fold increase in peak serum concentrations and area under the curve (AUC) following repeated monthly dosing during maintenance.
Population pharmacokinetic analysis showed that secukinumab was absorbed with an average absolute bioavailability of 73% in patients with plaque psoriasis. Across studies, absolute bioavailabilities in the range between 60 and 77% were calculated.
The bioavailability of secukinumab in PsA patients was 85% on the basis of the population pharmacokinetic model.
Following a single subcutaneous injection of 300 mg solution for injection in pre-filled syringe in plaque psoriasis patients, secukinumab systemic exposure was similar to what was observed previously with two injections of 150 mg.
Following subcutaneous administration of 300 mg at weeks 0, 1, 2, 3 and 4 followed by 300 mg every 2 weeks, the mean ± SD steady-state secukinumab trough concentration at week 16 was approximately 55.1±26.7 μg/ml and 58.1±30.1 μg/ml in HS study 1 and HS study 2, respectively.
Distribution
The mean volume of distribution during the terminal phase (Vz) following single intravenous administration ranged from 7.10 to 8.60 litres in plaque psoriasis patients, suggesting that secukinumab undergoes limited distribution to peripheral compartments.
Biotransformation
The majority of IgG elimination occurs via intracellular catabolism, following fluid-phase or receptor mediated endocytosis.
Elimination
Mean systemic clearance (CL) following a single intravenous administration to patients with plaque psoriasis ranged from 0.13 to 0.36 l/day. In a population pharmacokinetic analysis, the mean systemic clearance (CL) was 0.19 l/day in plaque psoriasis patients. The CL was not impacted by gender. Clearance was dose- and time-independent.
The mean elimination half-life, as estimated from population pharmacokinetic analysis, was 27 days in plaque psoriasis patients, ranging from 18 to 46 days across psoriasis studies with intravenous administration.
In a population pharmacokinetic analysis, the mean systemic CL following subcutaneous administration of 300 mg at weeks 0, 1, 2, 3, and 4 followed by 300 mg every 2 weeks to patients with hidradenitis suppurativa was 0.26 l/day.
The mean elimination half-life, as estimated from population pharmacokinetic analysis, was 23 days in hidradenitis suppurativa patients.
Linearity/non-linearity
The single and multiple dose pharmacokinetics of secukinumab in plaque psoriasis patients were determined in several studies with intravenous doses ranging from 1x 0.3 mg/kg to 3x 10 mg/kg and with subcutaneous doses ranging from 1x 25 mg to multiple doses of 300 mg. Exposure was dose proportional across all dosing regimens.
Special populations
Elderly patients
Based on population pharmacokinetic analysis with a limited number of elderly patients (n=71 for age ≥65 years and n=7 for age ≥75 years), clearance in elderly patients and patients less than 65 years of age was similar.
Patients with renal or hepatic impairment
No pharmacokinetic data are available in patients with renal or hepatic impairment. The renal elimination of intact secukinumab, an IgG monoclonal antibody, is expected to be low and of minor importance. IgGs are mainly eliminated via catabolism and hepatic impairment is not expected to influence clearance of secukinumab.
Effect of weight on pharmacokinetics
Secukinumab clearance and volume of distribution increase as body weight increases.
Paediatric population
Plaque psoriasis:
In a pool of the two paediatric studies, patients with moderate to severe plaque psoriasis (6 to less than 18 years of age) were administered secukinumab at the recommended paediatric dosing regimen. At week 24, patients weighing ≥25 and <50 kg had a mean ± SD steady-state trough concentration of 19.8 ± 6.96 μg/ml (n=24) after 75 mg of secukinumab and patients weighing ≥50 kg had mean ±SD trough concentration of 27.3 ± 10.1 μg/ml (n=36) after 150 mg of secukinumab. The mean ± SD steady-state trough concentration in patients weighing <25 kg (n=8) was 32.6 ± 10.8 μg/ml at week 24 after 75 mg dose.
Juvenile idiopathic arthritis:
In a paediatric study, ERA and JPsA patients (2 to less than 18 years of age) were administered secukinumab at the recommended paediatric dosing regimen. At week 24, patients weighing <50 kg, and weighing ≥50 kg had a mean ± SD steady-state trough concentration of 25.2±5.45 μg/ml (n=10) and 27.9±9.57 μg/ml (n=19), respectively.
Preclinical safety data
Non-clinical data revealed no special hazard for humans (adult or paediatric) based on conventional studies of safety pharmacology, repeated dose and reproductive toxicity, or tissue cross-reactivity.
Animal studies have not been conducted to evaluate the carcinogenic potential of secukinumab.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.