CRYSVITA Solution for injection Ref.[7654] Active ingredients: Burosumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Kyowa Kirin Holdings B.V., Bloemlaan 2, 2132NP Hoofddorp, The Netherlands, +31 (0) 237200822, medinfo@kyowakirin.com
Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for the treatment of bone diseases, other drugs affecting bone structure and mineralisation
ATC code: M05BX05
Mechanism of action
Burosumab is a recombinant human monoclonal antibody (IgG1) that binds to and inhibits the activity of fibroblast growth factor 23 (FGF23). By inhibiting FGF23, burosumab increases tubular reabsorption of phosphate from the kidney and increases serum concentration of 1,25 dihydroxy-Vitamin D.
Clinical efficacy in paediatric patients with XLH
Study UX023-CL301
In paediatric study UX023-CL301 61 patients aged 1 to 12 years (56% female; 44% male, Age at first dose, mean (SD): 6.3 (3.31) years) were randomised to burosumab (n=29) or active control (n=32; oral phosphate and active vitamin D). At entry to the study all patients had to have had a minimum of 6 months treatment of oral phosphate and active vitamin D. All patients had radiographic evidence of bone disease due to XLH (Rickets severity score ≥2). Burosumab was started at a dose of 0.8 mg/kg every 2 weeks and increased to 1.2 mg/kg if there was inadequate response, as measured by fasting serum phosphate. Those patients randomised to active control group received multiple daily doses of oral phosphate and active vitamin D.
The primary efficacy endpoint was the change in severity of rickets at Week 40, as assessed by the RGI- C (Radiographic Global Impression of change) score, compared between the burosumab and active control groups.
The RGI-C is a relative rating scale that compares a patient’s rickets before and after treatment utilising a 7-point ordinal scale to evaluate change in the same abnormalities rated in the RSS (as described below). Scores range from -3 (indicating severe worsening of rickets) to +3 (indicating complete healing of rickets).
The severity of paediatric rickets was measured using the RSS, a radiographic scoring method based on the degree of metaphyseal fraying, concavity, and the proportion of the growth plate affected. In the UX023-CL301 study, the RSS was scored using a predefined scale looking at specific abnormalities in the wrists and knees.
All patients (n=61) completed the 64 Week randomised Treatment Period. No patients had dose reductions and 8 (28%) of burosumab-treated patients received dose escalations to 1.2 mg/kg. A total of 51 patients entered the Treatment Extension Period, 26 patients in the active control→burosumab group 14 and 25 patients in the burosumab→burosumab group, and were treated with burosumab up to 124 Weeks.
Primary Efficacy Results
Greater healing of rickets at Week 40 was seen with burosumab treatment compared to active control and this effect was maintained at week 64, as shown in Figure 1. These results were sustained to Week 88 (n=21).
Figure 1. RGI-C Global Score (Mean ± SE) – Primary Efficacy Endpoint at Week 40 and 64 (Full Analysis Set):
Secondary Efficacy Results
Key Secondary efficacy endpoint results for Weeks 40 and 64 are presented in Table 3. These results were sustained to Week 88 (n=21).
Table 3. Secondary Efficacy Endpoint Results:
| Endpoint | Week | Active Control LS Mean (SE) | Burosumab LS Mean (SE) | Difference (burosumab – active control) |
|---|---|---|---|---|
| Lower Limb Deformity; assessed by RGI-C (GEE model) | 40 | +0.22 (0.080) | +0.62 (0.153) | +0.40 [95% CI: 0.07, 0.72] p=0.0162 |
| 64 | +0.29 (0.119) | +1.25 (0.170) | +0.97 [95% CI: +0.57, +1.37] p<0.0001 | |
| Height; Z-score | Baseline | -2.05 (0.87) | -2.32 (1.17) | |
| 40a | +0.03 (0.031) | +0.16 (0.052) | +0.12 [95% CI: 0.01, 0.24] p=0.0408 | |
| 64b | +0.02 (0.035) | +0.17 (0.066) | +0.14 [95% CI: 0.00, 0.29] p=0.0490 | |
| Rickets severity, RSS total Score | Baseline | 3.19 (1.141) | 3.17 (0.975) | |
| 40a | -0.72 (0.162) | -2.08 (0.104) | -1.34 [95% CI: 1.74, -0.94] p<0.0001 | |
| 64b | -1.01 (0.151) | -2.23 (0.117) | -1.21 [95% CI: -1.59, -0.83] p<0.0001 | |
| Serum ALP (U/L) | Baseline | 523 (154) | 511 (125) | |
| 40a | 489 (189) | 381 (99) | -97 [95% CI: -138, -56] p<0.0001 | |
| 64b | 495 (182) | 337 (86) | -147 [95% CI: -192, -102] p<0.0001 | |
| Six Minute Walk Test (m) | Baseline | 450 (106) | 385 (86) | |
| 40a | +4 (14) | +47 (16) | +43 [95% CI: -0.3, 87] p=0.0514 | |
| 64b | +29 (17) | +75 (13) | +46 [95% CI: 2, 89] p=0.0399 |
a the change from Baseline to Week 40 from ANCOVA model.
b the change from Baseline to Week 64 from GEE Model.
Serum Phosphate
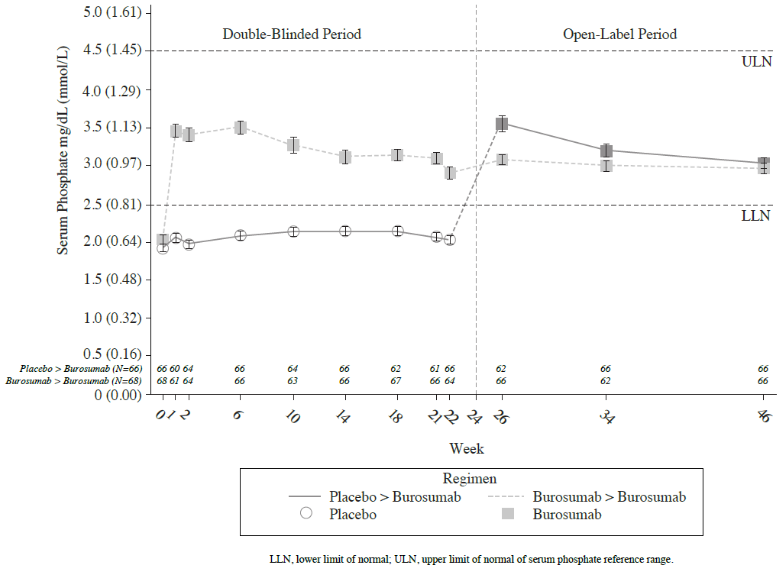
At each study visit at which serum phosphate was assessed in both groups, changes in serum phosphate from Baseline were larger in the burosumab group compared with the active control group (p<0.0001; GEE model) (Figure 2).
Figure 2. Serum Phosphate Concentration and Change from Baseline (mg/dL) (Mean ± SE) by Treatment Group (PD Analysis Set):
Note: Dashed line in figure indicates the lower limit of the normal serum phosphate reference range, 3.2 mg/dL (1.03 mmol/L)
During the Treatment Extension Period (Week 66 to Week 140), prolonger burosumab treatment in both groups (burosumab→burosumab (n=25) and active control→burosumab (n=26) the results were sustained.
Study UX023-CL201
In paediatric Study UX023-CL201, 52 paediatric patients aged 5 to 12 years (mean 8.5 years; SD 1.87) with XLH were treated for an initial period of 64 Weeks and dosed either every two weeks (Q2W) or every four weeks (Q4W). This was followed by two extension periods with dosing Q2W for all patients; the first period up to 96 Weeks (total 160 Weeks) and a further period of up to 56 Weeks for safety analysis.
Nearly all patients had radiographic evidence of rickets at baseline and had received prior oral phosphate and vitamin D analogues for a mean (SD) duration of 7 (2.4) years. This conventional therapy was discontinued 2-4 weeks prior to burosumab initiation. The burosumab dose was adjusted to target a fasting serum phosphate concentration of 3.50 to 5.02 mg/dL (1.13 to 1.62 mmol/L). In the first 64 Weeks, 26 of 52 patients received burosumab Q4W. Twenty six of 52 patients received burosumab Q2W at an average dose (min, max) of 0.73 (0.3, 1.5), 0.98 (0.4, 2.0) and 1.04 (0.4, 2.0) mg/kg at weeks 16, 40 and 64 respectively, and up to a maximum dose of 2.0 mg/kg.
Burosumab increased serum phosphate concentration and increased TmP/GFR. In the Q2W group, mean (SD) serum phosphate concentration increased from 2.38 (0.405) mg/dL (0.77 (0.131) mmol/L) at baseline), to 3.3 (0.396) mg/dL (1.07 (0.128) mmol/L) at Week 40 and was maintained to Week 64 at 17 3.35 (0.445) mg/dL (1.08 (0.144) mmol/L). The increased serum phosphate levels were sustained to Week 160 (n=52).
Alkaline phosphatase activity
Mean (SD) serum total alkaline phosphatase (ALP) activity was 459 (105) U/L at Baseline and decreased to 369 (76) U/L at Week 64 (-19.6%, p<0.0001); decreases were similar in the two dose groups. Overall, decreased serum ALP levels were sustained to Week 160.
Bone-derived serum alkaline phosphatase (BALP) content was 165 (52) μg/L [mean (SD)] at Baseline and 115 (31) μg/L at Week 64 (mean change: -28.5%); decreases were similar in the two dose groups. Overall, decreased serum BALP levels were sustained to Week 160.
In Study UX023-CL201 the severity of paediatric rickets was measured using the RSS, as described above, which was scored using a predefined scale looking at specific abnormalities in the wrists and knees. As a complement to the RSS assessment, the RGI-C rating scale was used. Results are summarised in Table 4.
Table 4. Rickets Response in Children 5-12 Years Receiving Burosumab in Study UX023-CL201:
| Endpoint | Duration of Burosumab (week) | Effect Size | |
|---|---|---|---|
| Q2W (N=26) | Q4W (N=26) | ||
| RSS Total Score Baseline Mean (SD) LS Mean change (SE) from baseline in total scorea (reduced RSS score indicates improvement in rickets severity) | 40 | 1.92 (1.2) -1.06 (0.100) (p<0.0001) | 1.67 (1.0) -0.73 (0.100) (p<0.0001) |
| 64 | -1.00 (0.1) (p<0.0001) | -0.84 (0.1) (p<0.0001) | |
| RGI-C Global Score LS Mean score (SE)a (positive indicates healing) | 40 | +1.66 (0.1) (p<0.0001) | +1.47 (0.1) (p<0.0001) |
| 64 | +1.56 (0.1) (p<0.0001) | +1.58 (0.1) (p<0.0001) | |
a The estimates of LS means and p-values are from the generalized estimation equation model accounting for baseline RSS, visits and regimen and its interaction.
Study UX023-CL205
In paediatric Study UX023-CL205, burosumab was evaluated in 13 XLH patients, aged 1 to 4 years (mean 2.9 years; SD 1.1) for a Treatment Period of 64 Weeks. Twelve patients continued to receive burosumab for an additional 96 Weeks during the Extension Period, for a maximum duration of 160 Weeks. All patients had radiographic evidence of rickets at baseline and 12 patients had received oral phosphate and vitamin D analogues for a mean (SD) duration of 16.7 (14.4) months. This conventional therapy was discontinued 2-6 weeks prior to burosumab initiation. Patients received burosumab at a dose of 0.8 mg/kg every two weeks.
Mean (SD) fasting serum phosphate concentration increased from 2.51 (0.284) mg/dL (0.81 (0.092) mmol/L) at baseline to 3.47 (0.485) mg/dL (1.12 (0.158) mmol/L) at Week 40 and the increased levels were sustained to Week 160.
Serum alkaline phosphatase activity
Mean (SD) serum total alkaline phosphatase activity was 549 (193.8) U/L at baseline and decreased to 335 (87.6) U/L at Week 40 (mean change: -36.3%). Decreased serum total alkaline phosphatase activity was sustained with long-term treatment to Week 160.
Rickets Severity Score (RSS)
Mean total RSS improved from 2.92 (1.367) at baseline to 1.19 (0.522) at Week 40, corresponding to a change from baseline in LS mean (SE) change of -1.73 (0.132) (p<0.0001). The RSS was sustained to Weeks 64, 112 and 160.
Radiographic Global Impression of Change (RGI-C)
After 40 weeks of treatment with burosumab, the LS mean (SE) RGI-C Global score was +2.21 (0.071) in all 13 patients (p < 0.0001) demonstrating healing of rickets. All 13 patients were considered RGI-C responders as defined by RGI-C global score ≥ +2.0. The RGI-C global score was sustained to Weeks 64, 112, and 160.
The European Medicines Agency has deferred the obligation to submit the results of studies with burosumab in one or more subsets of the paediatric population in treatment of X-linked hypophosphataemia. See 4.2 for information on paediatric use.
Clinical efficacy in adults with XLH
Study UX023-CL303
Study UX023-CL303 is a randomised, double-blind, placebo-controlled study in 134 adult XLH patients. The study comprised of a 24-week placebo-controlled treatment phase followed by a 24-week open-label period where all patients received burosumab. Oral phosphate and active vitamin D analogues were not allowed during the study. Burosumab was administered at a dose of 1 mg/kg every 4 weeks. The primary endpoint of this study was normalisation of serum phosphate across the 24-week double-blind period. Key secondary endpoints included worst pain as measured by the Brief Pain Inventory (BPI) scale and stiffness and physical function as measured by the WOMAC (Western Ontario and McMaster Universities Osteoarthritis) Index. Exploratory endpoints included fracture and pseudofracture healing, enthesopathy, 6 Minute Walk Test, BPI Pain interference, Brief Fatigue Inventory (BFI) worst fatigue and BFI global fatigue score.
At study entry, the mean age of patients was 40 years (range 19 to 66 years) and 35% were male. 66 patients were randomised to placebo treatment and 68 to burosumab treatment; at baseline, mean (SD) serum phosphate was 0.62 (0.10) mmol/L [1.92 (0.32) mg/dL] and 0.66 (0.1 mmol/L) [2.03 (0.30) mg/dL] in the placebo and burosumab groups respectively.
For the primary efficacy endpoint, a greater proportion of patients treated with burosumab achieved a mean serum phosphate level above the lower limit of normal (LLN) compared to the placebo group through week 24 (Table 5 and Figure 3).
Table 5. Proportion of Adult Patients Achieving Mean Serum Phosphate Levels Above the LLN at the Midpoint of the Dose Interval in Study UX023-CL303 (Double-Blind Period):
| Placebo (N=66) | Burosumab (N=68) | |
|---|---|---|
| Achieved Mean Serum Phosphate > LLN Across Midpoints of Dose Intervals Through Week 24 – n (%) | 7.6% (5/66) | 94.1% (64/68) |
| 95% CI | (3.3, 16.5) | (85.8, 97.7) |
| p-valuea | <0.0001 |
The 95% CIs are calculated using the Wilson score method.
a P-value is from Cochran-Mantel-Haenszel (CMH) testing for association between achieving the primary endpoint and treatment group, adjusting for randomisation stratifications.
Figure 3. Mean (± SE) Serum Phosphate Peak Concentrations (mg/dL [mmol/L]):
Patient reported pain, physical function and stiffness
Change from baseline at Week 24 showed a larger difference for burosumab relative to placebo in patient reported pain (BPI), physical function (WOMAC Index) and stiffness (WOMAC Index). The mean (SE) difference between treatment groups (burosumab-placebo) reach statistical significance for WOMAC stiffness at Week 24. Details are shown in Table 6.
Table 6. Patient reported pain, physical function and stiffness score changes from baseline to Week 24 and analysis of difference at Week 24:
| Placebo | Burosumab | |
|---|---|---|
| N=66 | N=68 | |
| BPI worst paina | ||
| LS Mean (SE) change from Baseline | -0.32 (0.2) | -0.79 (0.2) |
| [95% CIs] | [-0.76, 0.11] | [-1.20, -0.37] |
| LS Mean (SE) Difference (Burosumab-Placebo) | -0.5 (0.28) | |
| p-value | 0.0919c | |
| WOMAC Index physical functionb | ||
| LS Mean (SE) change from Baseline [95% CIs] | +1.79 (2.7) [-3.54, 7.13] | -3.11 (2.6) [-8.12, 1.89] |
| LS Mean (SE) Difference | -4.9 (2.5) | |
| p-value | 0.0478c | |
| WOMAC Index stiffnessb | ||
| LS Mean (SE) change from Baseline [95% CIs] | +0.25 (3.1) [5.89, 6.39] | -7.87 (3.0) [-13.82, -1.91] |
| LS Mean (SE) Difference (Burosumab-Placebo) | -8.12 (3.2) | |
| p-value | 0.0122 | |
a BPI worst pain item score ranges from 0 (no pain) to 10 (pain as bad as you can imagine)
b WOMAC Index physical function and stiffness domains range from 0 (best health) to 100 (worst health)
c Not significant following Hochberg adjustment
6 Minute Walk Test
This exercise test was conducted in all patients at Baseline, Week 12, 24, 36 and 48 (LS mean difference in change from baseline, burosumab → placebo; Table 7). Improvements continued through to Week 48 where distance walked increased from 357 m at baseline to 393 m at Week 48. Patients who crossed over from placebo to burosumab achieved similar improvements after 24 weeks of treatment.
Table 7. 6 Minute Walk distance (SD) Baseline and Week 24; Least Squares Mean Difference (SE):
| 6 MWT, m(SD) | Placebo | Burosumab |
|---|---|---|
| Baseline | 367 (103) | 357 (109) |
| Week 24 | 369 (103) | 382 (108) |
| LS Mean difference burosumab-placebo (SE) | 20 (7.7) | |
Radiographic Evaluation of Fractures and Pseudofractures
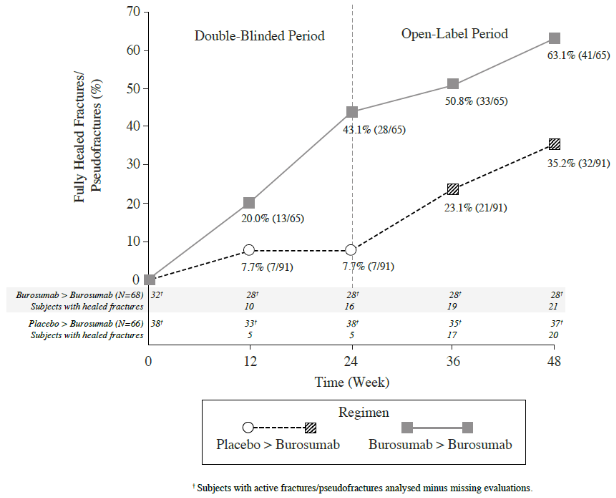
In Study UX023-CL303, a skeletal survey was conducted at baseline to identify osteomalacia-related fractures and pseudofractures. There were 52% (70/134) of patients who had either active fractures (12%, 16/134) or active pseudofractures (47%, 63/134) at baseline. Following burosumab treatment more patients showed healing of fractures and pseudofractures compared to the placebo group (Figure 4). During the placebo-controlled treatment period up to week 24, a total of 6 new fractures or pseudofractures appeared in 68 patients receiving burosumab compared to 8 new abnormalities in 66 patients receiving placebo. Of the number of new fractures developed prior to week 48 most (10/18) were healed or partially healed at the end of the study.
Figure 4. Percentage of Healed Active Fractures and Pseudofractures in Study UX023-CL303:
At Baseline, the mean (SD) total calcaneal enthesopathy burden (sum of superior and inferior calcaneal spurs) was 5.64 (3.12) cm in the burosumab group and 5.54 (3.1) cm in the placebo group. At Week 24, the mean (SD) total calcaneal enthesopathy burden was 5.90 (3.56) cm in the burosumab→burosumab group and 4.07 (2.38) cm in the placebo→burosumab group. For the exploratory endpoints of BPI Pain interference, BFI worst fatigue and BFI global fatigue score no meaningful difference were observed between treatment arms.
Bone Histomorphometry in Adults
Study UX023-CL304
Study UX023-CL304 is a 48-week, open-label, single-arm study in adult XLH patients to assess the effects of burosumab on improvement of osteomalacia as determined by histologic and histomorphometric evaluation of iliac crest bone biopsies. Patients received 1.0 mg/kg burosumab every 4 weeks. Oral phosphate and active vitamin D analogues were not allowed during the study.
14 patients were enrolled, and at study entry, the mean age of patients was 40 years (range 25 to 52 years) and 43% were male. After 48 weeks of treatment in Study UX023-CL304 paired biopsies were available from 11 patients; healing of osteomalacia was observed in all ten evaluable patients as demonstrated by decreases in osteoid volume/bone volume (OV/BV) from a mean (SD) score of 26.1% 22 (12.4) at baseline to 11.9% (6.6), Osteoid thickness (O.Th) declined in 11 evaluable patients from a mean (SD) of 17.2 (4.1) micrometres to 11.6 (3.1) micrometres.
Clinical Efficacy in adult patients with Tumour-induced osteomalacia
Burosumab has been evaluated in two single-arm open-label studies which enrolled a total of 27 adult patients with TIO. Oral phosphate and active vitamin D analogues were discontinued between 2-10 weeks before burosumab treatment was initiated. Patients received burosumab every 4 weeks at a weight based starting dose of 0.3 mg/kg to achieve a fasting serum phosphate level of 2.5 to 4.0 mg/dL [0.81 to 1.29 mmol/L].
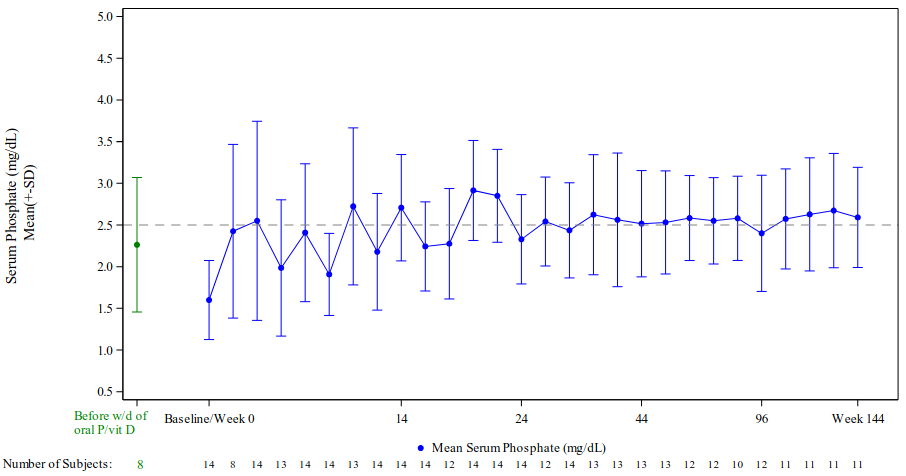
Study UX023T-CL201 enrolled 14 adult patients with a confirmed diagnosis of FGF23-related hypophosphataemia induced by an underlying tumour that was not amenable to surgical excision or could not be located. Eight patients were male and age range for all patients was from 33 years to 68 years of age (median 59.5 years). The mean (SD) dose of burosumab was 0.83 (0.41) mg/kg at Week 20, 0.87 (0.49) mg/kg at Week 48, 0.77 (0.52) mg/kg at Week 96 and 0.67 (0.54) mg/kg at Week 144.
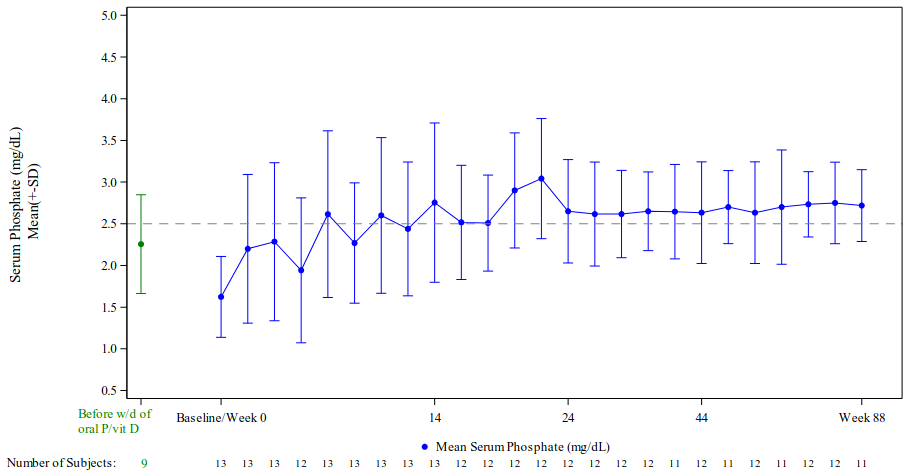
Study KRN23-002 enrolled 13 adult patients from Japan and South Korea with a confirmed diagnosis of TIO. Six patients were male and age range for all patients was from 41 years to 73 years of age (median 58.0 years). The mean (SD) dose of burosumab was 0.91 (0.59) mg/kg at Week 48, and 0.96 (0.70) mg/kg at Week 88.
Serum Phosphate
In both studies, burosumab increased mean serum phosphate levels and these remained stable throughout the study period, as shown in Figures 5 and 6, respectively.
Figure 5. Study UX023T-CL201 Serum Phosphate Concentration (mg/dL) (Mean ± SD):
Note: Dashed line in figure indicates the lower limit of the serum phosphate reference range, 2.5 mg/dL (0.81 mmol/L)
* Before withdrawal of oral phosphate/vitamin D; these values were taken before the enrolment in the study
Figure 6. Study KRN23-002 Serum Phosphate Concentration (mg/dL) (Mean ± SD):
Note: Dashed line in figure indicates the lower limit of the serum phosphate reference range, 2.5 mg/dL (0.81 mmol/L)
* Before withdrawal of oral phosphate/vitamin D; these values were taken before the enrolment in the study
In Study UX023T-CL201, the ratio of TmP/GFR increased in these patients from a mean (SD) of 1.12 (0.54) mg/dL [0.36 (0.17) mmol/L] at baseline to 2.12 (0.64) mg/dL [0.68 (0.21) mmol/L] at Week 48 and remained stable through to Week 144. In Study KRN23-002, the ratio of TmP/GFR, increased from a mean (SD) of 1.15 (0.43) mg/dL [0.46 (0.17) mmol/L] at baseline to 2.30 (0.48) mg/dL [0.92 (0.19) mmol/L] at Week 48.
Bone Histomorphometry
In Study UX023T-CL201, 11 patients had paired bone biopsies; changes were assessed after 48 weeks of treatment. Histomorphology parameters are presented below in Table 8 as group mean measurements at baseline and week 48, followed by the mean of relative changes of individualised measurements.
Table 8. Changes in histomorphology parameters in Study UX023T-CL201:
| Parameter | Group mean (SD) score | Percentage change in group mean values | |
|---|---|---|---|
| Baseline | Week 48 | ||
| OV/BV (%) | 17.6 (19.5) | 12.1 (15.4) | -31.3 |
| OS/BS (%) | 56.8 (31.0) | 56.6 (26.3) | -0.004 |
| O.Th (μm) | 16.5 (12.0) | 11.3 (9.2) | -31.5 |
Radiographic Evaluation
99m technetium-labelled whole body bone scans and x-ray skeletal surveys were conducted at baseline and post-treatment up to Week 144 to assess the number of fractures and pseudofractures. A reduction in fractures and pseudofractures was observed on both bone scans and x-rays.
Paediatric patients with TIO
There are no clinical trials with burosumab in paediatric patients of any age with TIO. The posology of burosumab in paediatric TIO patients has been determined from pharmacokinetic modelling and simulation (see section 5.2).
The European Medicines Agency has waived the obligation to submit the results of studies with burosumab in all subsets of the paediatric population in treatment of Tumour-induced Osteomalacia. See 4.2 for information on paediatric use.
Pharmacokinetic properties
Absorption
Burosumab absorption from subcutaneous injection sites to blood circulation is nearly complete. Following subcutaneous administration, the median time to reach maximum serum concentrations (Tmax) of burosumab is approximately 7-13 days. The peak serum concentration (Cmax) and area under the concentration-time curve (AUC) of serum burosumab is dose proportional over the dose range of 0.1-2.0 mg/kg.
Distribution
In XLH patients, the observed volume of distribution of burosumab approximates the volume of plasma, suggesting limited extravascular distribution.
Biotransformation
Burosumab is composed solely of amino acids and carbohydrates as a native immunoglobulin and is unlikely to be eliminated via hepatic metabolic mechanisms. Its metabolism and elimination are expected to follow the immunoglobulin clearance pathways, resulting in degradation to small peptides and individual amino acids.
Elimination
Due to its molecular size, burosumab is not expected to be directly excreted. The clearance of burosumab is dependent on body weight and estimated to be 0.290 L/day and 0.136 L/day in a typical adult (70 kg) and paediatric (30 kg) XLH patient, respectively, with corresponding disposition half-life (t1/2) in the serum ranging from approximately 16 to 19 days. Given the t1/2 estimates, the estimated time to reach the plateau of steady-state exposures is approximately 67 days. Following multiple dose administration to paediatric subjects, observed serum trough concentrations reach a plateau by 8 weeks after initiation of treatment.
Linearity/non-linearity
Burosumab displays time-invariant pharmacokinetics that is linear to dose over the subcutaneous dose range of 0.1 to 2.0 mg/kg.
Pharmacokinetic/pharmacodynamic relationship(s)
With the subcutaneous route of administration, in XLH and TIO subjects, a direct PK-PD relationship between serum burosumab concentrations and increases in serum phosphate concentration is observed and well described by an Emax/EC50 model. Serum burosumab and phosphate concentrations, as well as TmP/GFR, increased and decreased in parallel and reached maximum levels at approximately the same time point after each dose, supporting a direct PK-PD relationship. The AUC for the change from baseline in serum phosphate, TmP/GFR and 1,25(OH)2D increased linearly with increasing burosumab AUC.
Paediatric PK/PD
No significant difference has been observed in paediatric patient pharmacokinetics or pharmacodynamics as compared with PK/PD in the adult population. Burosumab clearance and volume of distribution are body weight dependent.
Paediatric patients with TIO
The starting dose of burosumab for paediatric patients with TIO is based on Population PK/PD modeling and simulations which indicate that a starting dose of 0.4 mg/kg every 2 weeks for children 25 aged 1-12 years and 0.3 mg/kg every 2 weeks for adolescents aged 13-17 years is predicted to result in a proportion of paediatric patients with TIO reaching normal serum phosphate levels. These can be titrated up to a maximum of 2.0 mg/kg every 2 weeks (the highest dose simulated).
Special Populations
Population PK analyses using data from paediatric and adult subjects who have XLH and adult subjects with TIO indicated that age, sex, race, ethnicity, baseline serum albumin, baseline serum alkaline phosphate, baseline serum alanine aminotransferase, and baseline creatinine clearance ≥49.9 mL/min, were not significant predictors of burosumab PK. Based on the population PK analysis, the PK characteristics of burosumab were similar between XLH and TIO patients.
Post-Prandial Effect on Serum Phosphate and Calcium
The effect of burosumab on serum phosphate and calcium levels after food was investigated in two sub-studies (Study UX023-CL301 and UX023-CL303); 13 paediatric patients (aged >3 years) and 26 adult patients (aged 24-65 years). Serum phosphate and calcium were measured at the end of the treatment interval in paediatric patients and mid-interval in adults. Blood samples were taken after a period of fasting, and again 1-2 hours after a standardised meal.
Burosumab treatment did not cause post-prandial excursions above the age-adjusted upper limits of normal in serum phosphate or serum calcium in any paediatric or adult subject in the sub-studies.
Preclinical safety data
Adverse reactions in non-clinical studies with normal animals were observed at exposures which resulted in serum phosphate concentration greater than normal limits. These effects were consistent with an exaggerated response to the inhibition of normal FGF23 levels resulting in a supraphysiologic increase in serum phosphate beyond the upper limit of normal.
Studies in rabbits and adult and juvenile cynomolgus monkeys demonstrated dose-dependent elevations of serum phosphate and 1,25 (OH)2D confirming the pharmacologic actions of burosumab in these species. Ectopic mineralisation of multiple tissues and organs (e.g. kidney, heart, lung, and aorta), and associated secondary consequences (e.g. nephrocalcinosis) in some cases, due to hyperphosphataemia, was observed in normal animals at doses of burosumab that resulted in serum phosphate concentrations in animals greater than approximately 8 mg/dL (2.6 mmol/L). In a murine model of XLH, a significant reduction in the incidence of ectopic mineralisation was observed at equivalent levels of serum phosphate, suggesting that the risk of mineralisation is less in the presence of excess FGF23.
Bone effects seen in adult and juvenile monkeys included changes in bone metabolism markers, increases in thickness and density of cortical bone, increased density of total bone and thickening of long bone. These changes were a consequence of higher than normal serum phosphate levels, which accelerated bone turnover and also led to periosteal hyperostosis and a decrease in bone strength in adult animals, but not in juvenile animals at the doses tested. Burosumab did not promote abnormal bone development, as no changes in femur length or bone strength were noted in juvenile animals. Bone changes were consistent with the pharmacology of burosumab and the role of phosphate in bone mineralization, metabolism and turnover.
In repeat-dose toxicology studies of up to 40 weeks duration in cynomolgus monkeys, mineralisation of the rete testis/seminiferous tubules was observed in male monkeys; however, no changes were observed in semen analysis. No adverse effects on female reproductive organs were observed in these studies.
In the reproductive and developmental toxicology study performed in pregnant cynomolgus monkeys, moderate mineralisation of the placenta was seen in pregnant animals given 30 mg/kg of burosumab and occurred in animals with peak serum phosphate concentration greater than approximately 8 mg/dL (2.6 mmol/L). Shortening of the gestation period and associated increased incidence of premature births were observed in pregnant monkeys at doses of ≥0.3 mg/kg which corresponded to burosumab exposures that are ≥0.875- to 1.39-fold anticipated clinical levels. Burosumab was detected in serum from fetuses indicating that burosumab was transported across the placenta to the fetus. There was no evidence of teratogenic effects. Ectopic mineralisation was not observed in foetuses or offspring and burosumab did not affect pre- and postnatal growth including survivability of the offspring.
In preclinical studies, ectopic mineralisation has been observed in normal animals, most frequently in the kidney, given burosumab at doses that resulted in serum phosphate concentrations greater than 8 mg/dL (2.6 mmol/L). Neither new or clinically meaningful worsening of nephrocalcinosis nor ectopic mineralisation have been observed in clinical trials of patients with XLH treated with burosumab to achieve normal serum phosphate levels.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.