CYRAMZA Concentrate for solution for infusion Ref.[8882] Active ingredients: Ramucirumab
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Eli Lilly Nederland B.V., Papendorpseweg 83, 3528 BJ Utrecht, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, monoclonal antibodies
ATC code: L01XC21
Mechanism of action
Vascular Endothelial Growth Factor (VEGF) Receptor 2 is the key mediator of VEGF induced angiogenesis. Ramucirumab is a human receptor-targeted antibody that specifically binds VEGF Receptor 2 and blocks binding of VEGF-A, VEGF-C, and VEGF-D. As a result, ramucirumab inhibits ligand stimulated activation of VEGF Receptor 2 and its downstream signalling components, including p44/p42 mitogen-activated protein kinases, neutralising ligand-induced proliferation and migration of human endothelial cells.
Clinical efficacy and safety
Gastric cancer
RAINBOW
RAINBOW, a global, randomised, double-blind, study of Cyramza plus paclitaxel versus placebo plus paclitaxel, was conducted in 665 patients with locally recurrent and unresectable or metastatic gastric cancer (including GEJ adenocarcinoma) following platinum- and fluoropyrimidine-containing chemotherapy, with or without anthracycline. The primary endpoint was overall survival (OS) and the secondary endpoints included progression free survival (PFS) and overall response rate (ORR). Patients were required to have experienced disease progression during, or within 4 months after the last dose of first-line therapy and with ECOG PS 0-1. Patients were randomised in a 1:1 ratio to receive Cyramza plus paclitaxel (n=330) or placebo plus paclitaxel (n=335). Randomisation was stratified by geographic region, time to progression from the start of first-line therapy (<6 months versus ≥6 months) and disease measurability. Cyramza at 8 mg/kg or placebo was administered by intravenous infusion every 2 weeks (on days 1 and 15) of a 28-day cycle. Paclitaxel at 80 mg/m 2 was administered by intravenous infusion on days 1, 8, and 15 of each 28-day cycle.
A majority (75%) of patients randomised in the study received prior platinum and fluoropyrimidine combination therapy without anthracycline. The remainder (25%) received prior platinum and fluoropyrimidine combination therapy with anthracycline. Two-thirds of the patients experienced disease progression while still on first-line therapy (66.8%). Baseline patient demographics and disease characteristics were generally balanced between arms: the median age was 61 years; 71% of patients were male; 61% were Caucasian, 35% Asian; the ECOG PS was 0 for 39% of patients, 1 for 61% of patients; 81% of patients had measurable disease and 79% had gastric cancer; 21% had GEJ adenocarcinoma. The majority of patients (76%) had experienced disease progression within 6 months from the start of first-line therapy. For patients treated with Cyramza plus paclitaxel the median duration of therapy was 19 weeks, and for patients treated with placebo plus paclitaxel the median duration of therapy was 12 weeks. The median relative dose intensity of Cyramza was 98.6% and of placebo was 99.6%. The median relative dose intensity of paclitaxel was 87.7% for the Cyramza plus paclitaxel arm and 93.2% for the placebo plus paclitaxel arm. A similar percentage of patients discontinued treatment due to adverse events: 12% of patients treated with Cyramza plus paclitaxel compared with 11% of patients treated with placebo plus paclitaxel. Post discontinuation systemic anti-cancer therapy was given to 47.9% of patients receiving Cyramza plus paclitaxel and 46.0% of patients receiving placebo plus paclitaxel.
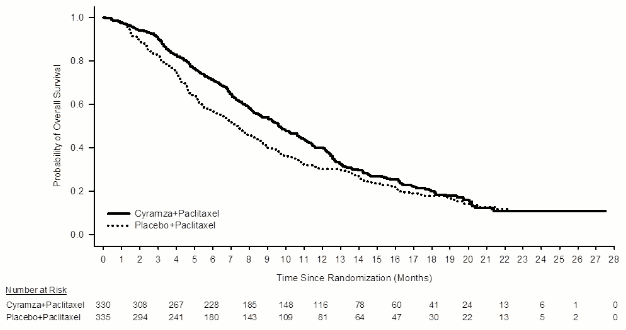
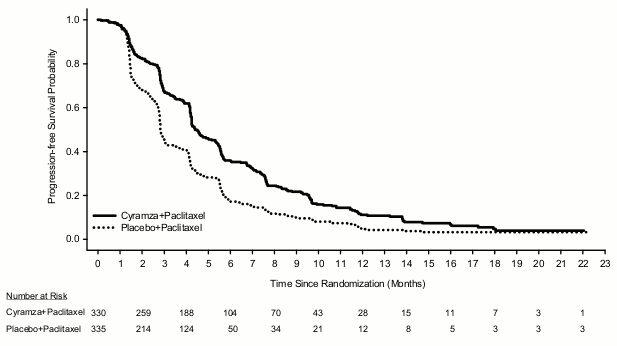
Overall survival was statistically significantly improved in patients receiving Cyramza plus paclitaxel compared with those receiving placebo plus paclitaxel (HR 0.807; 95% CI: 0.678 to 0.962; p=0.0169). There was an increase in median survival of 2.3 months in favour of the Cyramza plus paclitaxel arm: 9.63 months in the Cyramza plus paclitaxel arm and 7.36 months in the placebo plus paclitaxel arm. Progression-free survival was statistically significantly improved in patients receiving Cyramza plus paclitaxel compared with those receiving placebo plus paclitaxel (HR 0.635; 95% CI: 0.536 to 0.752; p<0.0001). There was an increase in median PFS of 1.5 months in favour of the Cyramza plus paclitaxel arm: 4.4 months in the Cyramza plus paclitaxel arm and 2.9 months in the placebo plus paclitaxel arm. Objective response rate [ORR] was significantly improved in patients receiving Cyramza plus paclitaxel compared with those receiving placebo plus paclitaxel (Odds ratio 2.140; 95% CI: 1.499 to 3.160; p=0.0001). The ORR in the Cyramza plus paclitaxel arm was 27.9% and in the placebo plus paclitaxel arm was 16.1%. Improvements in OS and PFS were consistently observed in pre-specified subgroups based on age, sex, race and in most other pre-specified subgroups. Efficacy results are shown in Table 8.
Table 8. Summary of efficacy data – Intent to treat (ITT) population:
| Cyramza plus paclitaxel N=330 | Placebo plus paclitaxel N=335 | |
|---|---|---|
| Overall survival, months | ||
| Median (95% CI) | 9,6 (8,5, 10,8) | 7,4 (6,3, 8,4) |
| Hazard ratio (95% CI) | 0,807 (0,678, 0,962) | |
| Stratified log-rank p-value | 0,0169 | |
| Progression free survival, months | ||
| Median (95% CI) | 4,4 (4,2, 5,3) | 2,9 (2,8, 3,0) |
| Hazard ratio (95% CI) | 0,635 (0,536, 0,752) | |
| Stratified log-rank p-value | <0,0001 | |
| Objective response rate (CR +PR) | ||
| Rate-percent (95% CI) | 27,9 (23,3, 33,0) | 16,1 (12,6, 20,4) |
| Odd ratio | 2,140 (1,449, 3,160) | |
| Stratified CMH p-value | 0,0001 | |
Abbreviations: CI = confidence interval, CR= complete response, PR= partial response, CMH= Cochran-Mantel-Haenszel
Figure 1. Kaplan-Meier curves of overall survival for Cyramza plus paclitaxel versus placebo plus paclitaxel in RAINBOW:
Figure 2. Kaplan-Meier curves of progression-free survival for Cyramza plus paclitaxel versus placebo plus paclitaxel in RAINBOW:
REGARD
REGARD, a multinational, randomised, double-blind study of Cyramza plus BSC versus placebo plus BSC, was conducted in 355 patients with locally recurrent and unresectable, or metastatic gastric cancer (including GEJ adenocarcinoma) following platinum- or fluoropyrimidine-containing chemotherapy. The primary endpoint was OS and secondary endpoints included PFS. Patients were required to have experienced disease progression during, or within 4 months after the last dose of, first-line therapy for metastatic disease, or during adjuvant treatment or within 6 months after the last dose of adjuvant therapy, and had ECOG PS 0-1. To be included in the study, patients were required to have total bilirubin of ≤1.5 mg/dl and AST and ALT ≤3 times ULN, or ≤5 times ULN if liver metastases were present.
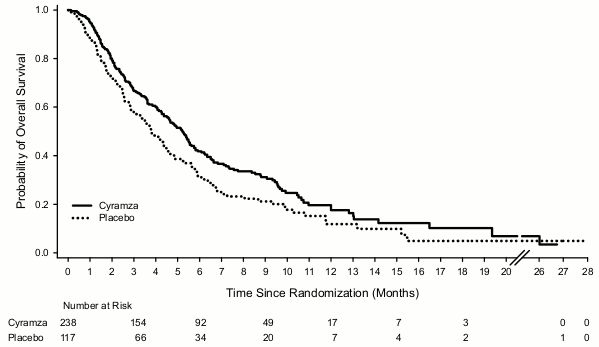
Patients were randomised in a 2:1 ratio to receive an intravenous infusion of Cyramza 8 mg/kg (n= 238) or placebo (n= 117) every 2 weeks. Randomisation was stratified by weight loss over the prior 3 months (≥10% versus <10%), geographic region, and location of the primary tumour (gastric versus GEJ). Baseline demographics and disease characteristics were balanced. The ECOG PS was 1 for 72% of patients. There were no patients with Child-Pugh B or C liver cirrhosis enrolled in REGARD. 11% of patients treated with Cyramza and 6% of patients on placebo discontinued therapy due to adverse events. Overall survival was statistically significantly improved in patients receiving Cyramza as compared with patients receiving placebo (hazard ratio [HR] 0.776; 95%CI: 0.603 to 0.998; p=0.0473), corresponding to a 22% reduction in the risk of death and an increase in median survival to 5.2 months for Cyramza from3.8 months for placebo. Progression-free survival was statistically significantly improved in patients receiving Cyramza as compared with patients receiving placebo (HR 0.483; 95%CI: 0.376 to 0.620; p<0.0001), corresponding to a 52% reduction in the risk of progression or death and an increase in median PFS to 2.1 months for Cyramza from 1.3 months for placebo. Efficacy results are shown in Table 9.
Table 9. Summary of efficacy data – ITT population:
| Cyramza N=238 | Placebo N=117 | |
|---|---|---|
| Overall survival, months | ||
| Median (95% CI) | 5,2 (4,4, 5,7) | 3,8 (2,8, 4,7) |
| Hazard ratio (95% CI) | 0,776 (0,603, 0,998) | |
| Stratified log-rank p-value | 0,0473 | |
| Progression free survival, months | ||
| Median (95% CI) | 2,1 (1,5, 2,7) | 1,3 (1,3, 1,4) |
| Hazard ratio (95% CI) | 0,483 (0,376, 0,620) | |
| Stratified log-rank p-value | <0,0001 | |
| 12-week PFS rate% (95% CI) | 40,1 (33,6, 46,4) | 15,8 (9,7, 23,3) |
Abbreviations: CI = confidence interval
Figure 3. Kaplan-Meier curves of overall survival for Cyramza versus placebo in REGARD:
Based on limited data from REGARD patients with HER2-positive gastric or GEJ adenocarcinoma and patients previously treated with trastuzumab (in RAINBOW), it is considered unlikely that Cyramza has a detrimental effect or that it has no effect in patients with HER2-positive gastric cancer. Post hoc unstratified subgroup analyses from RAINBOW patients previously treated with trastuzumab (n=39) suggested a survival benefit in such patients (HR 0.679, 95% CI 0.327, 1.419) and demonstrated a benefit for progression free survival (PFS) (HR 0.399, 95% CI 0.194, 0.822).
Colorectal cancer
RAISE
RAISE was a global, randomised, double-blind, study of Cyramza plus FOLFIRI versus placebo plus FOLFIRI, in patients with mCRC, who had disease progression on or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine. Patients were required to have ECOG PS 0 or 1 and to have disease progression within 6 months of the last dose of first-line therapy. Patients were required to have adequate hepatic, renal and coagulation function. Patients with a history of uncontrolled hereditary or acquired bleeding or thrombotic disorders, a recent history of severe (Grade ≥3) bleeding or who had experienced an arterial thrombotic event (ATE) in the 12 months prior to randomisation were excluded. Patients were also excluded if they had experienced any of: an ATE, Grade 4 hypertension, Grade 3 proteinuria, a grade 3-4 bleeding event, or bowel perforation during first-line bevacizumab therapy.
A total of 1072 patients were randomised (1:1) to receive either Cyramza (n=536) at 8 mg/kg or placebo (n=536), in combination with FOLFIRI. All medicinal products were administered intravenously. The FOLFIRI regimen was: irinotecan 180 mg/m 2 administered over 90 minutes and folinic acid 400 mg/m² administered, simultaneously over 120 minutes; followed by bolus 5-fluorouracil(5-FU) 400 mg/m² over 2 to 4 minutes; followed by 5-FU 2400 mg/m² administered by continuous infusion over 46 to 48 hours. Treatment cycles on both arms were repeated every 2 weeks. Patients who discontinued one or more components of treatment because of an adverse event were permitted to continue therapy with the other treatment component(s) until disease progression or unacceptable toxicity. The primary endpoint was OS and the secondary endpoints included PFS, objective response rate (ORR) and quality of life (QoL) using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30. Randomisation was stratified by geographic region, tumour KRAS status (mutant or wild-type), and time to disease progression (TTP) after commencing first-line treatment (<6 months versus ≥6 months).
Demographic and baseline characteristics for the ITT population were similar between treatment arms. Median age was 62 years and 40% of patients were ≥65 years; 57% of patients were male; 76% were White and 20% Asian; 49% had ECOG PS 0; 49% of patients had KRAS mutant tumours; and 24% of patients had TTP <6 months after commencing first-line treatment. Post discontinuation systemic anti- cancer therapy was given to 54% of patients receiving Cyramza plus FOLFIRI and 56% of patients receiving placebo plus FOLFIRI.
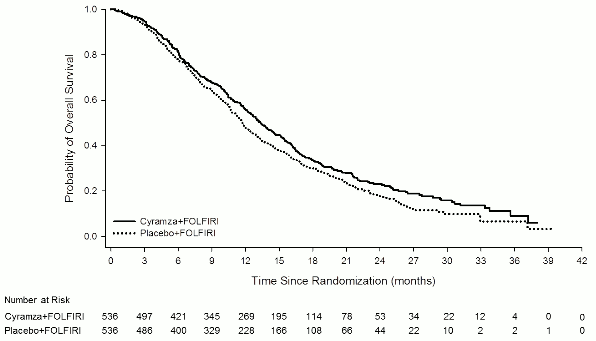
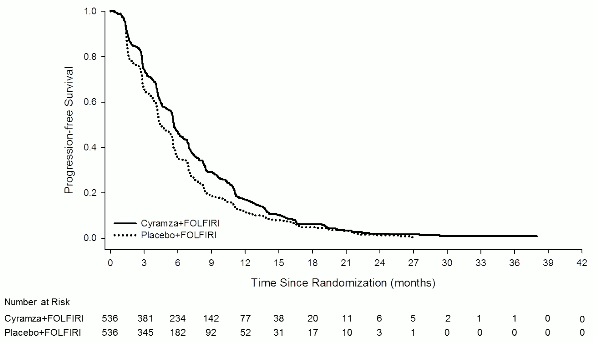
Overall survival was statistically significantly improved in patients receiving Cyramza plus FOLFIRI compared with those receiving placebo plus FOLFIRI (HR 0.844; 95% CI: 0.730 to 0.976; p=0.0219). There was an increase in median survival of 1.6 months in favour of the Cyramza plus FOLFIRI arm: 13.3 months in the Cyramza plus FOLFIRI arm and 11.7 months in the placebo plus FOLFIRI arm. Progression-free survival was statistically significantly improved in patients receiving Cyramza plus FOLFIRI compared with those receiving placebo plus FOLFIRI (HR 0.793; 95% CI: 0.697 to 0.903; p=0.0005). There was an increase in median PFS of 1.2 months in favour of the Cyramza plus FOLFIRI arm: 5.7 months in the Cyramza plus FOLFIRI arm and 4.5 months in the placebo plus FOLFIRI arm. Efficacy results are shown in Table 10 and Figures 4 and 5.
Pre-specified analyses for OS and PFS by stratification factors were performed. The HR of OS was 0.82 (95% CI: 0.67 to 1.0) in patients with a KRAS wild type tumour, and 0.89 (95% CI: 0.73 to 1.09) in patients with a KRAS mutant tumour. For patients with TTP ≥6 months after commencing first-line treatment the HR of OS was 0.86 (95% CI: 0.73 to 1.01), and 0.86 (95% CI: 0.64 to 1.13) in patients with TTP <6 months after commencing first-line treatment. Pre-specified subgroup analyses for both PFS and OS according to age (<65 and ≥65 years), gender, race, ECOG PS (0 or ≥1), number of organs involved, liver metastases only, site of primary tumour (colon or rectum), carcinoembryonic antigen levels (<200 μg/L, ≥200 μg/L), all showed a treatment effect favouring Cyramza plus FOLFIRI treatment over placebo plus FOLFIRI. In 32 of the 33 pre-specified sub-group analyses for OS, the HR was < 1.0. The one sub-group with HR >1 was for patients with disease progression from start of first-line bevacizumab treatment of <3 months (HR 1.02 [95% CI: 0.68 to 1.55]). This one sub-group is a group which can be considered to have aggressive disease that is relatively refractory to first-line treatment. In both treatment arms, patients who experienced neutropenia had a longer median OS compared to patients who did not experience neutropenia. The median OS in patients with any grade neutropenia was greater in the ramucirumab arm (16.1 months) than in the placebo arm (12.6 months). Median OS in patients who did not experience neutropenia was 10.7 months in both arms.
Table 10. Summary of efficacy data – ITT population:
| Cyramza plus FOLFIRI N=536 | Placebo plus FOLFIRI N=536 | |
|---|---|---|
| Overall survival, months | ||
| Median (95% CI) | 13,3 (12,4, 14,5) | 11,7 (10,8, 12,7) |
| Hazard ratio (95% CI) | 0,84 (0,73, 0,98) | |
| Stratified log-rank p-value | 0,022 | |
| Ελεύθερη εξέλιξης νόσου επιβίωση, μήνες | ||
| Median (95% CI) | 5,7 (5,5, 6,2) | 4,5 (4,2, 5,4) |
| Hazard ratio (95% CI) | 0,79 (0,70, 0,90) | |
| Stratified log-rank p-value | <0,001 | |
Abbreviations: CI = confidence interval
Figure 4. Kaplan-Meier curves of overall survival for Cyramza plus FOLFIRI versus placebo plus FOLFIRI in RAISE:
Figure 5. Kaplan-Meier curves of progression -free survival for Cyramza plus FOLFIRI versus placebo plus FOLFIRI in RAISE:
The ORR was similar for both treatment arms (13.4% versus 12.5%, ramucirumab plus FOLFIRI versus placebo plus FOLFIRI, respectively). The disease control rate (complete response plus partial response plus stable disease) was numerically higher in patients on the ramucirumab plus FOLFIRI arm as compared to the placebo plus FOLFIRI arm (74.1% versus 68.8%, respectively). For the EORTC QLQ-C30, patients in the ramucirumab plus FOLFIRI treatment arm reported a transient decrease in QoL compared to the patients in the placebo plus FOLFIRI treatment arm in most of the scales. Few between-arm differences were reported after the first month of treatment.
NSCLC
RELAY
RELAY was a global, randomised, double-blind, phase 3 study of Cyramza plus erlotinib versus placebo plus erlotinib that randomised (1:1) 449 previously untreated patients with metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 (L858R) activating mutations at study entry. Eligible patients were ECOG PS 0 or 1. Patients with CNS metastases or known T790M EGFR mutations at baseline were excluded from the study. Patients at a high risk of bleeding, cardiovascular events, including those who had experienced any arterial thrombotic event within 6 months of enrolment, were also excluded from the study.
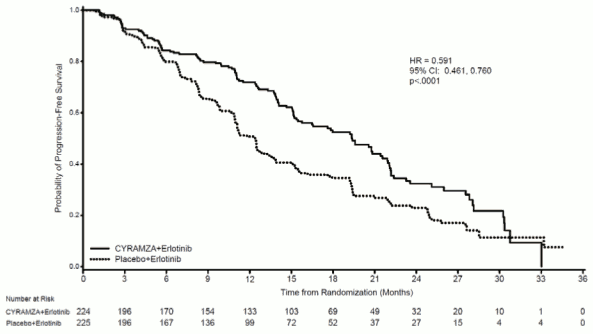
Demographics and baseline characteristics were balanced between arms. 77% of patients were Asian and 22% were Caucasian. Patients treated with Cyramza plus erlotinib experienced a statistically significant improvement in progression-free survival (PFS) compared to patients treated with placebo plus erlotinib (Table 11). Consistent results were observed across subgroups including exon 19 deletions and exon 21 (L858R) substitution, age, race (Caucasian HR: 0.618, Asian HR: 0.638), smokers and never smokers. Overall survival data were immature at the time of the final PFS analysis (17.6% maturity). RELAY efficacy results are shown in Table 11 and Figure 6.
Table 11. Summary of efficacy data in RELAY – Intent to treat (ITT) population:
| Cyramza plus erlotinib N=224 | Placebo plus erlotinib N=225 | |
|---|---|---|
| Progression-free Survival | ||
| Number of events (%) | 122 (54,5) | 158 (70,2) |
| Median – months (95% CI) | 19,4 (15,38, 21,55) | 12,4 (10,97, 13,50) |
| Hazard Ratio (95% CI) | 0,591 (0,461, 0,760) | |
| Stratified Log-rank p-value | <0,0001 | |
| Interim Overall Survival | ||
| Number of events (%)<>37 (16,5) | 42 (18,7) | |
| Median – months (95% CI) | NR | NR |
| Hazard Ratio (95% CI) | 0,832 (0,532, 1,303) | |
| Stratified Log-rank p-value | 0,4209 | |
| Objective Response Rate (Complete Response + Partial Response) | ||
| Ποσοστό % (95% CI) | 76 (70,8, 81,9) | 75 (69,0, 80,3) |
| CR, Ν (%) | 3 (1,3) | 2 (0,9) |
| PR, Ν (%) | 168 (75,0) | 166 (73,8) |
| Duration of Response | N=171 | N=168 |
| Number of events (%) | 101 (59,1) | 128 (76,2) |
| Median – months (95% CI) | 18,0 (13,86, 19,78) | 11,1 (9,69, 12,29) |
| Hazard Ratio (95% CI) | 0,619 (0,477, 0,805) | |
| Stratified Log-rank p-value | 0,0003 | |
Abbreviations: CI = confidence interval, NR= not reached, CR = complete response, PR = partial response. A hierarchal testing procedure was employed to test OS. OS was tested only if PFS was significant. Both endpoints were alpha-protected.
Figure 6. Kaplan-Meier curves of progression free survival for Cyramza plus erlotinib versus placebo plus erlotinib in RELAY:
REVEL
REVEL, a randomised, double-blind study of Cyramza plus docetaxel versus placebo plus docetaxel, was conducted in 1253 patients with locally advanced or metastatic squamous or non-squamous NSCLC with disease progression on or after one platinum-based therapy. The primary endpoint was OS. Patients were randomised in a 1:1 ratio to receive Cyramza plus docetaxel (n=628) or placebo plus docetaxel (n=625). Randomisation was stratified by geographic region, gender, prior maintenance, and ECOG PS. Cyramza at 10 mg/kg or placebo and docetaxel at 75 mg/m² were each administered by intravenous infusion on day 1 of a 21-day cycle. Sites in East Asia administered a reduced dose of docetaxel at 60 mg/m 2 every 21 days. Patients with recent serious pulmonary, gastrointestinal, or postoperative bleeding, evidence of CNS haemorrhage, tumour involvement of major airway or blood vessel, intra-tumour cavitation, and history of significant bleeding or uncontrolled thrombotic disorders were excluded. Also, patients receiving any kind of therapeutic anticoagulation and/or chronic therapy with non-steroidal anti-inflammatory drugs or other anti-platelets agents or those with untreated, clinically unstable brain/CNS metastases were excluded Aspirin use at doses up to 325 mg/day was permitted. (see section 4.4). A limited number of non-Caucasian, especially Black patients (2.6%) were included. Therefore there is limited experience with the combination of ramucirumab and docetaxel in these patients with advanced NSCLC as well as in patients with renal impairment, cardiovascular disease and obesity.
Baseline patient demographics and disease characteristics were generally balanced between arms: the median age was 62 years; 67% of patients were male; 82% were Caucasian, 13% Asian; the ECOG PS was 0 for 32% of patients, 1 for 67% of patients; 73% of patients had non-squamous histology and 26% had squamous histology. The most common prior therapies included pemetrexed (38%), gemcitabine (25%), taxane (24%), and bevacizumab (14%); 22% of patients received prior maintenance therapy. The median duration of docetaxel therapy was 14.1 weeks for the ramucirumab plus docetaxel arm (with a median of 4.0 infusions received) and 12.0 weeks for the placebo plus docetaxel arm (with a median of 4.0 infusions received).
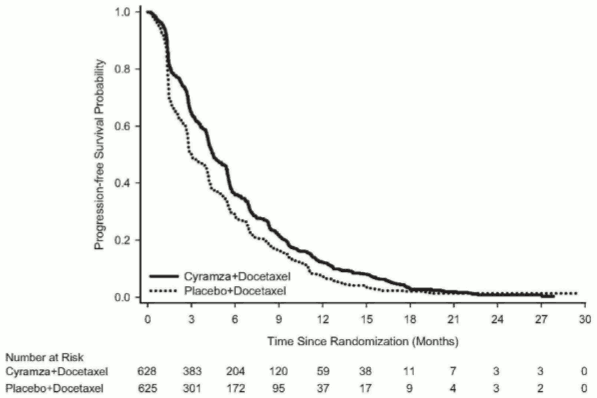
OS was statistically significantly improved in patients receiving Cyramza plus docetaxel compared with those receiving placebo plus docetaxel (HR 0.857; 95% CI: 0.751 to 0.979; p=0.024). There was an increase in median survival of 1.4 months in favour of the Cyramza plus docetaxel arm: 10.5 months in the Cyramza plus docetaxel arm and 9.1 months in the placebo plus docetaxel arm. PFS was statistically significantly improved in patients receiving Cyramza plus docetaxel compared with those receiving placebo plus docetaxel (HR 0.762; 95% CI: 0.677 to 0.859; p<0.001). There was an increase in median PFS of 1.5 months in favour of the Cyramza plus docetaxel arm: 4.5 months in the Cyramza plus docetaxel arm and 3 months in the placebo plus docetaxel arm. ORR was significantly improved in patients receiving Cyramza plus docetaxel compared with those receiving placebo plus docetaxel (22.9% vs. 13.6%, p<0.001). The primary QoL analysis showed similar time to deterioration for all Lung Cancer Symptom Scale (LCSS) scores between treatment arms.
A consistent improvement (ramucirumab plus docetaxel vs placebo plus docetaxel) was observed in important subgroups for PFS and OS. OS subgroup results included the following: non-squamous histology (HR 0.83; 95% CI: 0.71 to 0.97; median OS [mOS]: 11.1 vs 9.7 months) and squamous histology (HR 0.88; 95% CI: 0.69 to 1.13; mOS: 9.5 vs 8.2 months); patients with prior maintenance (HR 0.69; 95% CI: 0.51 to 0.93; mOS: 14.4 vs 10.4 months); time since start of prior therapy <9 months (HR 0.75; 95% CI: 0.64 to 0.88; mOS: 9.3 vs 7.0 months); patients <65 years old (HR 0.74, 95% CI: 0.62, 0.87; mOS: 11.3 vs 8.9 months). A trend towards less efficacy with increasing age has been observed in patients receiving ramucirumab plus docetaxel for the treatment of advanced NSCLC with disease progression after platinum-based chemotherapy (see section 5.1). No differences in efficacy between treatment arms have been observed in the subgroups of patients ≥65 years old (OS HR 1.10, 95% CI: 0.89, 1.36; median OS [mOS]: 9.2 vs 9.3 months, see section 4.4), patients pre-treated with taxanes (HR 0.81; 95% CI:0.62 to 1.07; mOS 10.8 vs 10.4 months) and those with time since start of prior therapy ≥9 months (HR 0.95; 95% CI: 0.75 to 1.2; mOS: 13.7 vs 13.3 months). Efficacy results are shown in Table 12.
Table 12. Summary of efficacy data – ITT population:
| Cyramza plus docetaxel N=628 | Placebo plus docetaxel N=625 | |
|---|---|---|
| Overall survival, months | ||
| Median – months (95% CI) | 10,5 (9,5, 11,2) | 9,1 (8,4, 10,0) |
| Hazard Ratio (95% CI) | 0,857 (0,751, 0,979) | |
| Stratified log-rank p-value | 0,024 | |
| Progression free survival, months | ||
| Median (95% CI) | 4,5 (4,2, 5,4) | 3,0 (2,8, 3,9) |
| Hazard Ratio (95% CI) | 0,762 (0,677, 0,859) | |
| Stratified log-rank p-value | <0,001 | |
| Objective response rate (CR + PR) | ||
| Rate – percent (95% CI) | 22,9 (19,7, 26,4) | 13,6 (11,0, 16,5) |
| Stratified CMH p-value | <0,001 | |
Abbreviations: CI = confidence interval, CR= complete response, PR= partial response, CMH = Cochran-Mantel-Haenszel
Figure 7. Kaplan-Meier curves of overall survival for Cyramza plus docetaxel versus placebo plus docetaxel in REVEL:
Figure 8. Kaplan-Meier curves of progression-free survival for Cyramza plus docetaxel versus placebo plus docetaxel in REVEL:
Hepatocellular carcinoma
REACH-2
REACH-2 was a global, randomised, double-blind study of Cyramza plus BSC versus placebo plus BSC that randomised (2:1) 292 patients with HCC who had a serum AFP ≥400 ng/ml at study entry. Patients enrolled into the study had disease progression on or after prior sorafenib therapy or were intolerant to sorafenib. Eligible patients were Child Pugh A (score <7), had creatinine clearance ≥60 ml/min, and ECOG PS of 0 or 1. In addition, patients were either Barcelona Clinic Liver Cancer (BCLC) stage B and no longer amenable to locoregional therapy, or were BCLC stage C. Patients with brain metastases, leptomeningeal disease, uncontrolled spinal cord compression, a history of or current hepatic encephalopathy or clinically meaningful ascites, severe variceal bleeding in the 3 months prior to treatment, or gastric or oesophageal varices at high risk of bleeding were excluded from the study. The primary endpoint was overall survival. The threshold for the elevated AFP study entry requirement for REACH-2 was determined based on the survival results from a pre-specified subgroup, exploratory analysis from REACH, a previously completed, supportive phase 3 clinical study in 565 HCC patients randomised (1:1) to either Cyramza plus BSC or placebo plus BSC that had disease progression on or after prior sorafenib therapy.
In REACH-2, baseline patient demographics and disease characteristics were generally balanced between arms, except for AFP, which was lower in the placebo arm. Patients treated with Cyramza experienced a statistically significant improvement in OS, compared to placebo (Table 13). The major efficacy outcome in REACH-2 was supported by a statistically significant improvement in progression free survival in Cyramza treated patients compared to placebo treated patients. The relative treatment effect (assessed by HR) of Cyramza compared to placebo was generally consistent across subgroups, including age, race, aetiology of disease and reason for discontinuation of sorafenib (progressive disease vs. intolerance). A relevant exposure-efficacy association was observed for ramucirumab in REACH-2 (see section 5.2). REACH-2 efficacy results are shown in Table 13 and Figure 9.
Table 13. Summary of efficacy data in REACH-2 – Intent to treat (ITT) population:
| Cyramza N=197 | Placebo N=95 | |
|---|---|---|
| Overall survival, months | ||
| Median (95% CI) | 8,51 (7,00, 10,58) | 7,29 (5,42, 9,07) |
| Hazard ratio (95% CI) | 0,710 (0,531, 0,949) | |
| Stratified log-rank p-value | 0,0199 | |
| Progression free survival, months | ||
| Median (95% CI) | 2,83 (2,76, 4,11) | 1,61 (1,45, 2,69) |
| Hazard ratio (95% CI) | 0,452 (0,339, 0,603) | |
| Stratified log-rank p-value | <0,0001 | |
| Objective Response Rate (CR + PR) | ||
| ORR % (95% CI) | 4,6 (1,7, 7,5) | 1,1 (0,0, 3,1) |
| p-value | 0,1697 | |
Figure 9. Kaplan-Meier curves of Overall Survival for Cyramza versus placebo in REACH-2:
Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) ≥2 patients
Patients with ECOG score ≥2 were excluded from the pivotal studies in all indications, therefore the safety and efficacy of Cyramza in this patient population is unknown.
Immunogenicity
Patients in two Phase 3 studies, RAINBOW and REGARD were tested at multiple time-points for anti-drug antibodies (ADAs). Samples were tested from 956 patients: 527 ramucirumab treated patients and 429 control treated patients. Eleven (2.2%) of ramucirumab treated patients and two (0.5%) of control treated patients developed ADAs. None of the patients with ADAs experienced an IRR. No patients had neutralising antibodies to ramucirumab. There is insufficient data to evaluate the effects of ADAs on the efficacy or safety of ramucirumab.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Cyramza in all subsets of the paediatric population in gastric adenocarcinoma, in adenocarcinoma of the colon and rectum, in lung carcinoma, and liver cancer (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Following the dose regimen of 8 mg/kg every 2 weeks, the geometric means of ramucirumab Cmin prior to administration of the fourth and seventh dose of ramucirumab given as a single agent in advanced gastric cancer patients' serum were 49.5 μg/ml (range of 6.3-228 μg/ml) and 74.4 μg/ml (range of 13.8-234 μg/ml), respectively. In HCC patients' serum the geometric means of ramucirumab Cmin prior to administration of the second, fourth and seventh dose of ramucirumab were 23.5 μg/ml (range of 2.9-76.5 μg/ml), 44.1 μg/ml (range of 4.2-137 μg/ml) and 60.2 μg/ml (range of 18.3-123 μg/ml), respectively.
Following the dose regimen of 8 mg/kg ramucirumab every 2 weeks in combination with FOLFIRI, the geometric means of ramucirumab Cmin were 46.3 μg/ml (range of 7.7-119 μg/ml) and 65.1 μg/ml (range of 14.5-205 μg/ml) prior to administration of the third and fifth dose, respectively, in serum from patients with mCRC.
Following the dose regimen of 10 mg/kg ramucirumab every 3 weeks, the geometric means of ramucirumab Cmin were 28.3 μg/ml (range of 2.5-108 μg/ml) and 38.4 μg/ml (range of 3.1-128 μg/ml) prior to administration of the third and fifth dose, respectively of ramucirumab given in combination with docetaxel, in serum from patients with NSCLC.
Following the dose regimen of 10 mg/kg ramucirumab every 2 weeks, the geometric means of ramucirumab Cmin were 68.5 μg/ml (range of 20.3-142 μg/ml) and 85.7 μg/ml (range of 36.0-197 μg/ml) prior to administration of the fourth and seventh dose, respectively of ramucirumab given in combination with erlotinib, in serum from patients with NSCLC.
Absorption
Cyramza is administered as an intravenous infusion. There have been no studies performed with other routes of administration.
Distribution
Based on population pharmacokinetic approach (PopPK), the mean (% coefficient of variation [CV%]) volume of distribution at steady state for ramucirumab was 5.4L (15%).
Biotransformation
The metabolism of ramucirumab has not been studied. Antibodies are principally cleared by catabolism.
Elimination
Based on PopPK, the mean (CV%) clearance of ramucirumab was 0.015 L/hour (30%) and the mean half-life was 14 days (20%).
Time and dose dependency
There was no clear deviation from dose proportionality in pharmacokinetics of ramucirumab from 6 mg/kg to 20 mg/kg. An accumulation ratio of 1.5 was observed for ramucirumab when dosed every 2 weeks. Based on simulations using the PopPK model, steady state would be attained by the sixth dose.
Elderly
Based on PopPK, there was no difference in ramucirumab exposure in patients ≥65 years of age compared to patients <65 years old.
Renal impairment
No formal studies have been conducted to evaluate the effect of renal impairment on the pharmacokinetics of ramucirumab. Based on PopPK, ramucirumab exposure was similar in patients with mild renal impairment (creatinine clearance [CrCl] ≥60 to <90 ml/min), moderate renal impairment (CrCl ≥30 to <60 ml/min) or severe renal impairment (CrCl 15 to 29 ml/min) as compared to patients with normal renal function (CrCl ≥90 ml/min).
Hepatic impairment
No formal studies have been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of ramucirumab. Based on PopPK, ramucirumab exposure in patients with mild hepatic impairment (total bilirubin >1.0-1.5 upper limit of normal (ULN) and any AST or total bilirubin ≤1.0 ULN and AST >ULN) or moderate hepatic impairment (total bilirubin >1.5-3.0 ULN and any AST) was similar to patients with normal hepatic function (total bilirubin and AST ≤ ULN). Ramucirumab has not been studied in patients with severe hepatic impairment (total bilirubin >3.0 ULN and any AST).
Other special populations
Based on PopPK, the following covariates were found to have no impact on ramucirumab disposition: age, sex, race, albumin levels. These and other factors investigated had <20% effect on ramucirumab disposition. Body weight is considered a significant co-variate of ramucirumab pharmacokinetics supporting the dosing based on body weight.
Exposure response relationships
Efficacy
Exposure-response analyses indicated that efficacy was correlated with ramucirumab exposure across pivotal studies. Efficacy, as measured by improvements in OS, was associated with increasing ramucirumab exposure range produced by 8 mg/kg ramucirumab given every 2 weeks and by 10 mg/kg ramucirumab given every 3 weeks. An improvement in PFS was also associated with increasing ramucirumab exposure for advanced gastric cancer, NSCLC with disease progression after platinum-based chemotherapy and mCRC.
In the REACH-2 study for HCC, a relevant exposure-efficacy association was observed for ramucirumab which showed that only patients with above-median exposure experienced an improvement in OS, compared to placebo, and these exposure-efficacy relationships remained after attempts to adjust for other prognostic factors. A treatment effect on PFS was observed for all exposure levels produced by 8 mg/kg ramucirumab given every 2 weeks. No such relation was observed in the RELAY study for NSCLC with 10 mg/kg ramucirumab plus erlotinib given every 2 weeks.
Safety
In RAINBOW, the incidences of Grade ≥3 hypertension, neutropenia, and leukopenia were increased with higher ramucirumab exposure.
In RAISE, the incidence of Grade ≥3 neutropenia was increased with higher ramucirumab exposure.
In RELAY, no exposure-safety relationship was identified for the selected safety endpoints, including Grade ≥3 hypertension, diarrhoea, proteinuria and dermatitis acneiform.
In REVEL, the incidences of Grade ≥3 febrile neutropenia and hypertension were increased with higher ramucirumab exposure.
In the pooled data from REACH-2 and REACH (patients with alpha fetoprotein ≥400 ng/ml), the incidences of Grade ≥3 hypertension was increased with higher ramucirumab exposure.
Preclinical safety data
No animal studies have been performed to test ramucirumab for potential of carcinogenicity or genotoxicity.
The target organs identified in repeated dose cynomolgus monkey toxicity studies were kidney (glomerulonephritis), bone (thickening and abnormal endochondral ossification of the epiphyseal growth plate) and female reproductive organs (decreased weight of ovaries and uterus). A minimal grade of inflammation and/or mononuclear cell infiltration was seen in several organs.
Reproductive toxicity studies with ramucirumab have not been performed, however, animal models link angiogenesis, VEGF and VEGF Receptor 2 to critical aspects of female reproduction, embryo-foetal development, and postnatal development. Based on ramucirumab’s mechanism of action, it is likely that in animals, ramucirumab will inhibit angiogenesis and result in adverse effects on fertility (ovulation), placental development, developing foetuses and postnatal development.
A single dose of ramucirumab did not impair wound healing in monkeys using a full-thickness incisional model.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.