CYSTAGON Capsule Ref.[27453] Active ingredients: Mercaptamine
Source: FDA, National Drug Code (US) Revision Year: 2019
2. Clinical Pharmacology
Mechanism of Action
Cystinosis is an autosomal recessive inborn error of metabolism in which the transport of cystine out of lysosomes is abnormal; in the nephropathic form, accumulation of cystine and formation of crystals damage various organs, especially the kidney, leading to renal tubular Fanconi Syndrome and progressive glomerular failure, with end stage renal failure by the end of the first decade of life. In four studies of cystinosis patients before cysteamine was available, renal death (need for transplant or dialysis) occurred at median age of less than 10 years. Patients with cystinosis also experience growth failure, rickets, and photophobia due to cystine deposits in the cornea. With time most organs are damaged, including the retina, muscles and central nervous system.
Cysteamine is an aminothiol that participates within lysosomes in a thiol-disulfide interchange reaction converting cystine into cysteine and cysteine-cysteamine mixed disulfide, both of which can exit the lysosome in patients with cystinosis.
Pharmacokinetics and Pharmacodynamics
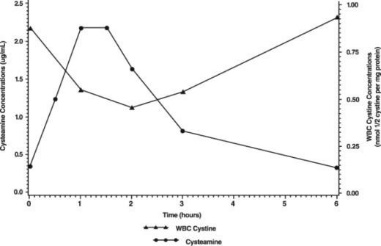
Normal individuals and persons heterozygous for cystinosis have white cell cystine levels of <0.2 and usually below 1 nmol/½ cystine/mg protein, respectively. Individuals with nephropathic cystinosis have elevations of white cell cystine above 2 nmol/½ cystine/mg protein. White cell cystine is monitored in these patients to determine adequacy of dosing, levels being measured 5 to 6 hours after dosing. In the Long-Term Study (see Clinical Trials, below) entry white cell cystine levels were 3.73 nmol/½ cystine/mg protein (range 0.13 to 19.80 nmol/½ cystine/mg protein) and were maintained close to 1 nmol/½ cystine/mg protein with a cysteamine dose range of 1.3 to 1.95 g/m²/day. After administration of cysteamine HCl, leukocyte cystine levels fall, with minimum levels at approximately 1 hour.
Because cysteamine HCl has an unpleasant taste and odor, other formulations have been developed, including phosphocysteamine, the phosphorothioester of cysteamine that is rapidly converted to cysteamine in the gut, and cysteamine bitartrate (CYSTAGON). Cysteamine bitartrate has been shown in a transfer study in 8 patients to maintain white cell cystine levels below 1 nmol/½ cystine/mg protein when substituted for cysteamine HCl or phosphocysteamine. Total cysteamine levels 2 and 6 hours post-dosing were higher after cysteamine bitartrate than for the solutions.
The pharmacokinetics and pharmacodynamics of CYSTAGON were studied in eleven pediatric patients with nephropathic cystinosis who received 225 to 550 mg of cysteamine bitartrate every 6 hours daily for more than one year. Following repeated oral administration of 225 to 550 mg cysteamine bitartrate, the mean time to maximum plasma concentration (Tmax) occurred at about 1.4 hours post dose with mean steady-state peak plasma concentration (Cmax) and area under the concentration-time curve (AUC) of 2.6 µg/mL and 6.3 µg∙hr/mL, respectively. The apparent volume of distribution and apparent plasma clearance of cysteamine were 156 L and 1.2 L/min, respectively.
Cysteamine was moderately bound to human plasma proteins, predominantly to albumin, with mean protein binding of about 52%. Plasma protein binding was independent of concentration over the concentration range achieved clinically with the recommended doses.
The pharmacodynamic response increased with the plasma cysteamine concentration, with maximum response occurring approximately 1.8 hours post dose with an average reduction of white cell cystine concentration of about 0.46 nmol/½ cystine/mg protein and returning to baseline level 6 hours post dose.
The mean white cell cystine concentration and mean cysteamine plasma concentration-time profile is shown below:
Most clinical data have been developed using cysteamine HCl or phosphocysteamine solutions. In all discussions that follow, administered amounts of various cysteamine salts will be expressed as amounts of cysteamine free base.
6.6. Carcinogenesis, Mutagenesis, Impairment of Fertility
Cysteamine has not been tested for its carcinogenic potential in long-term animal studies.
Cysteamine was not mutagenic in the Ames test. It produced a negative response in an in-vitro sister chromatid exchange assay in human lymphocytes, but a positive response in a similar assay in hamster ovarian cells.
Repeat breeding reproduction studies were conducted in male and female rats. Cysteamine was found to have no effect on fertility and reproductive performance at an oral dose of 75 mg/kg/day (450 mg/m²/day, 0.4 times the recommended human dose based on body surface area). At an oral dose of 375 mg/kg/day (2,250 mg/m²/day, 1.7 times the recommended human dose based on body surface area), it reduced the fertility of the adult rats and the survival of their offspring.
13. Clinical Studies
There are approximately 200 pre-transplant cystinosis patients in the United States with nephropathic cystinosis and clinical studies have included almost all of them, in addition to about 40 studied in the United Kingdom. For all patients, mean age at entry into studies was just under 4 years. Patients were approximately equally divided between genders and about 85% were white, 9% were black, and 3% were hispanic.
The National Collaborative Cysteamine Study (NCCS) treated 94 children (mainly from the United States) with nephropathic cystinosis with increasing doses of cysteamine HCl (mean dose 54 mg/kg/day) to attain white cell cystine levels of less than 2 nmol/½ cystine/mg protein 5 to 6 hours post-dose, and compared their outcome with an historical control group of 17 children who had been in the placebo group of a randomized placebo-controlled trial of ascorbic acid. Cysteamine treated patients had been diagnosed at a mean age of 22 months and were a mean age of 46 months old at study entry; placebo patients had been diagnosed at about 29 months and were a mean age of about 52 months old at study entry. The principal measures of effectiveness were serum creatinine and calculated creatinine clearance and growth (height).
The average median white cell cystine level attained during treatment in the NCCS was 1.7 ± 0.2 nmol/½ cystine/mg protein. There were 70 cysteamine patients with baseline serum creatinine less than 2 mg/dL who were followed for at least a year and 17 placebo patients. Twelve of the 94 cysteamine treated patients required early dialysis or renal transplant. Median follow-up of cysteamine patients was over 32 months and 20% were followed more than 5 years. For the placebo group median follow-up was 20 months and only one was followed more than 24 months. Among cysteamine patients glomerular function was maintained over time despite the longer period of treatment and follow-up. Placebo treated patients, in contrast, experienced a gradual rise in serum creatinine. Height, corrected for age, was compared for treated patients with the height, at the various ages patients appeared, of the 143 patients initially screened for inclusion in the NCCS. Patients on treatment maintained growth (did not show increasing growth failure compared to normals) although growth velocity did not increase enough to allow patients to catch up to age norms. Renal tubular function was not affected by treatment.
Calculated creatinine clearances were evaluated for two groups of children, one with poor white cell cystine depletion and one with good white cell cystine depletion as shown in Table I. The final mean creatinine clearance of the good depletion group was 20.8 ml/min/1.73 m² greater than the mean for the poor depletion group, despite the older mean age of the good depletion group.
TABLE 1. CREATININE CLEARANCE CHANGES BY WHITE CELL CYSTINE DEPLETION:
| Age (years) | Creatinine Clearance (ml/min/1.73m²) | |||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Poor Depletion* (n=18) | 4.2 ± 1.8 | 6.5 ± 0.5 | 33.6 ± 17.0 | 29.7 ± 5.4 |
| Good Depletion† (n=19) | 3.3 ± 2.2 | 7.2 ± 0.7 | 44.3 ± 15.0 | 50.5 ± 5.1 |
(95% confidence limits on difference between final creatinine clearances, 6.1 and 35.5 ml/min/1.73m², despite older mean age of good depletion group.)
* Median leukocyte cystine levels were over 3 nmol/½ cystine/mg protein or were not measured at least 2 times per year. Patients did receive cysteamine for at least 1 year.
† Median leukocyte cystine levels were less than 1 nmol/½ cystine/mg protein and received cysteamine for at least 1 year.
The Long Term Study, initiated in 1988, utilized both cysteamine HCl and phosphocysteamine (patient’s choice) in 46 patients who had completed the NCCS (averaging 6.5 years of treatment) and 93 new patients. Patients had cystinosis diagnosed by elevated white cell cystine (mean 3.63 nmol/½ cystine/mg). New patients and 46 continuing patients were required to have serum creatinine less than 3.0 mg/dL and 4.0 mg/dL, respectively. Patients were randomized to doses of 1.3 or 1.95 g/m²/day and stratified according to whether the serum creatinine was above 1.2 mg/dL or not. Doses could be raised if white cell cystine levels were approximately 2 nmol/½ cystine/mg protein and lowered due to intolerance.
Mean doses were 1.27 g/m²/day and 1.87 g/m²/day in the two groups and white cell cystine levels averaged 1.72 ± 1.65 nmol/½ cystine/mg protein and 1.86 ± 0.92 nmol/½ cystine/mg protein in the 1.3 and 1.95 g/m²/day groups, respectively. In new patients, a group similar in age to the NCCS group, serum creatinine was essentially unchanged over the period of follow-up (about half of the patients were followed for 24 months) and phosphocysteamine and cysteamine HCl had similar effects. The long-term follow-up group, about nine years old on average at entry, stayed in the study (almost 80% were followed at least 2 years) and had essentially no change in renal function. In four studies of untreated cystinosis, renal death (need for transplant or dialysis) occurred at median age of less than 10 years. Both groups maintained height (although they did not catch up from baseline). There was no apparent difference between the two doses.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.