DALIDUO Film-coated tablet Ref.[50512] Active ingredients: Dolutegravir Rilpivirine
Source: Health Products Regulatory Authority (ZA) Revision Year: 2022 Publisher: CIPLA MEDPRO (PTY) LTD, Building 9, Parc du Cap, Mispel Street, Bellville, 7530 Phone: +27 21 943 4200 Customer Care: 080 222 6662 E-mail: info@cipla.co.za
4.3. Contraindications
- Hypersensitivity to rilpivirine, dolutegravir or any of the excipients in DALIDUO.
- It is not recommended that DALIDUO be co-administered with other Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI’s) e.g. delavirdine, efavirenz, etravirine, nevirapine, see section 4.5.
- DALIDUO should not be used in combination with carbamazepine, oxcarbazepine, phenobarbitone, phenytoin and systemic dexamethasone (except as a single dose treatment), see section 4.5.
- Rifabutin, rifampicin and rifapentine are potent inducers of CYP3A enzymes. DALIDUO should not be used in combination with these medicines, see section 4.5.
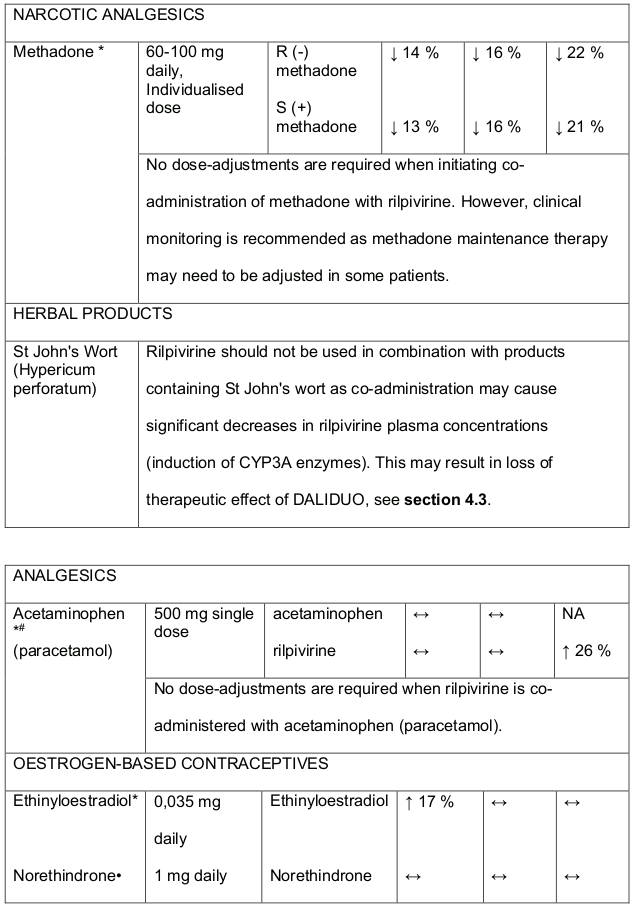
- DALIDUO should not be used concomitantly with products containing St. Johns Wort, see section 4.5.
- The proton-pump inhibitors (PPIs) lansoprazole, omeprazole, rabeprazole, pantoprazole and esomeprazole should not be administered concomitantly with DALIDUO, see section 4.5.
- DALIDUO is contraindicated in combination with dofetilide and pilsicainide.
- DALIDUO is contraindicated in moderate and severe hepatic impairment.
- Fampridine (also known as dalfampridine).
- Peri-conception, during the first trimester of pregnancy and in lactation.
4.4. Special warnings and precautions for use
Safety and efficacy of the individual active ingredients in various ART combination regimens with similar dosages as contained in DALIDUO have been established in clinical studies for the treatment of HIV patients. However, safety and efficacy of the actives combined in a fixed drug combination (FDC) as in DALIDUO have not been established.
Hypersensitivity reactions have been reported with integrase inhibitors and were characterised by rash, constitutional findings and sometimes organ dysfunction, including liver injury. Discontinue DALIDUO and other suspect medicines immediately if signs or symptoms of hypersensitivity reactions develop (including, but not limited to severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial oedema, hepatitis, eosinophilia, angioedema). Clinical status including liver aminotransferases should be monitored and appropriate therapy initiated. Delay in stopping treatment with DALIDUO or other suspect medicines after the onset of hypersensitivity may result in a life–threatening reaction.
Patients should be advised that current antiretroviral therapy does not cure HIV and has not been proven to prevent the transmission of HIV to others through blood, other bodily secretion or sexual contact. Appropriate precautions to prevent transmission of HIV should continue to be employed.
Virologic Failure and Development of Resistance
In a study, patients treated with rilpivirine with a baseline viral load >100, 000 HIV-1 RNA copies/mL had a greater risk of virologic failure compared to patients with a baseline viral load ≤100, 000 HIV-1 RNA copies/mL. Patients with a baseline viral load >100, 000 HIV-1 RNA copies/ml who experienced virologic failure exhibited a higher rate of treatment-emergent resistance to the NNRTI class. Patients who failed virologically on rilpivirine-containing products developed lamivudine/ emtricitabine-associated resistance. This information should be taken into consideration when initiating therapy with DALIDUO.
Interaction with other medicines
Caution should be given to prescribing DALIDUO with other medicines that may reduce the exposure of rilpivirine, see section 4.5. There is limited information available on the potential for a pharmacodynamic interaction between rilpivirine and medicines that prolong the QTc interval of the electrocardiogram. In a study of healthy subjects, supratherapeutic doses of rilpivirine (75 mg daily and 300 mg daily) have been shown to prolong the QTc interval of the electrocardiogram, see section 5.1 and 4.5. DALIDUO should be used with caution when co-administered with medicines with a known risk of Torsade de Pointes.
Fat distribution
Redistribution/accumulation of body fat, including central obesity, dorsocervical fat enlargement (buffalo lump), peripheral wasting, facial wasting, breast enlargement, cushingoid appearance and elevated serum lipid and glucose levels have been observed in HIV patients and those receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established. Clinical examination should include evaluation for physical signs of fat redistribution. Patients with evidence of lipodystrophy should have a thorough cardiovascular risk assessment.
Immune reconstitution syndrome
Immune reconstitution syndrome has been reported in HIV–infected patients with severe immune deficiency at the time of initiation of antiretroviral therapy. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium complex, cytomegalovirus, Pneumocystis jiroveci (P. carinii) pneumonia and tuberculosis). This inflammatory response and the underlying infections may necessitate further evaluation and treatment which should not be delayed. Auto-immune disorders (such as Graves disease, polymyositis and Guillain-Barre syndrome) have also reported to occur in the setting of immune reconstitution, however the time to onset is more variable and can occur many months after initiation of treatment and sometimes can be an atypical presentation. Liver chemistry elevations consistent with immune reconstitution syndrome were observed in some hepatitis B and/or C co-infection. Monitoring of liver chemistries is recommended in patients with hepatitis B and/or C co infection. Particular diligence should be applied in initiating or maintaining effective hepatitis B therapy (referring to treatment guidelines) when starting dolutegravir-based therapy in hepatitis B coinfected patients, see section 4.8.
Osteonecrosis
Although the aetiology is considered to be multifactorial (including corticosteroid use, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have reported particularly in patients with advanced HIVdisease and/ or long-term exposure to combination antiretroviral therapy (cART). patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Hepatic impairment
The unbound fraction of dolutegravir in the blood is doubled in patients with moderate hepatic impairment. DALIDUO is contraindicated in patients with moderate or severe hepatic impairment, see section 4.3.
Opportunistic infections
Patients should be advised that DALIDUO does not cure HIV infection and that they may still develop opportunistic infections and other complications of HIV infection and should be closely monitored.
Patients with hepatitis B or C
There is no clinical data available in patients with hepatitis B co-infection. Medical practitioners should refer to current treatment guidelines for the management of HIV infection in patients co-infected with hepatitis B virus. Limited data is available in patients with hepatitis C co-infection. A higher incidence of liver chemistry elevations (Grade 1) may occur in patients treated with dolutegravir and rilpivirine co-infected with hepatitis C compared to those who were not coinfected. Monitoring of liver function is recommended in patients with hepatitis B and/or C co-infection. In patients with advanced liver or cirrhosis, treatment discontinuation is not recommended since post – treatment exacerbation of hepatitis may lead to hepatic decompensation.
DALIDUO contains sugars/sweeteners such as mannitol which may have a mild
laxative effect and lactose monohydrate. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine.
4.5. Interaction with other medicinal products and other forms of interaction
DALIDUO contains dolutegravir and rilpivirine and any interactions that have been identified with either component individually, may occur with DALIDUO. There are no significant interactions between dolutegravir and rilpivirine. Recommendations are based on either interaction studies or predicted interactions due to expected magnitude of interaction and/or potential for serious effects or loss of potency. DALIDUO is not expected to be co-administered with other HIV-1 antiretroviral medicines and information is provided for reference. Dolutegravir interactions:
In vitro, dolutegravir had no direct, or weak inhibition (IC50>50 µm) of the enzymes cytochrome P450 (CYP)1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A, uridine diphosphate glucuronosyl transferase (UGT) 1A1 or UGT2B7, or the transporters Pgp, BCRP, OATP1B1, OCT1 or MRP2. In vitro, dolutegravir did not induce CYP1A2, CYP2B6 or CYP3A4. In vivo, dolutegravir did not have an effect on midazolam, a CYP3A4 probe. Based on these data, dolutegravir is not expected to affect the pharmacokinetics of medicines that are substrates of these enzymes or transporters (e.g. reverse transcriptase and protease inhibitors, opioid analgesics, antidepressants, statins, azole antifungals (such as fluconazole, itraconazole, clotrimazole), proton pump inhibitors (such as esomeprazole, lansoprazole, omeprazole), anti-erectile dysfunction medicines (such as sildenafil, tadalafil, vardenafil), aciclovir, valaciclovir, sitagliptin, adefovir).
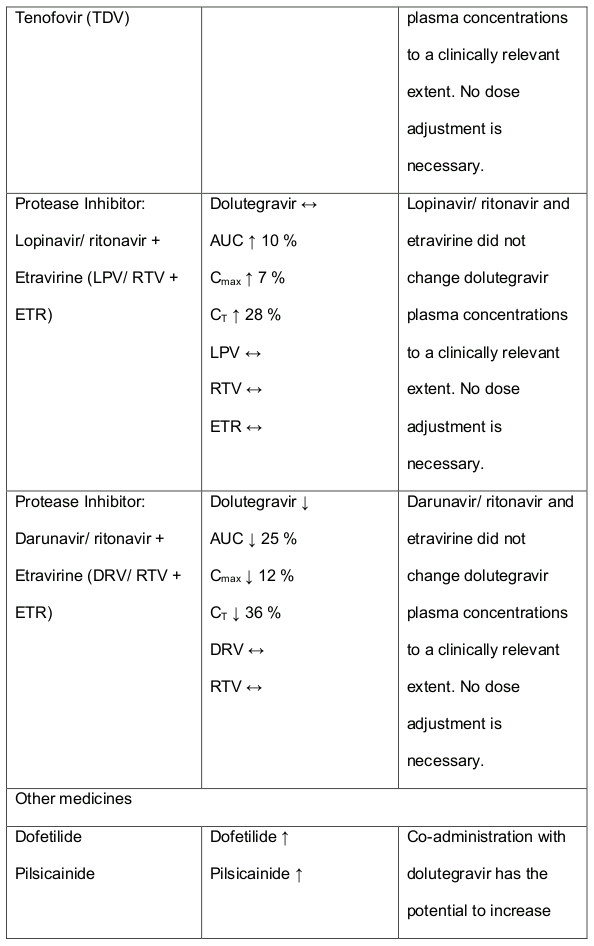
In medicine interaction studies, dolutegravir did not have a clinically relevant effect on the pharmacokinetics of the following: tenofovir, methadone, efavirenz, lopinavir, atazanavir, darunavir, etravirine, fosamprenavir, rilpivirine, telaprevir and oral contraceptives containing norgestimate and ethyl estradiol. In vitro, dolutegravir inhibited the renal organic cation transporter 2 (OCT2). Base this observation, dolutegravir a component of DALIDUO may increase plasma concentrations of medicines in which excretion is dependent upon OCT2 (dofetilide, pilsicainide or metformin). See table 1 below: Medicine Interactions – Other Medicines).
Effect of other medicines on the pharmacokinetics of dolutegravir
Dolutegravir is eliminated mainly through metabolism by UGT 1A1. Dolutegravir is also a substrate of UGT1A3, UGT1A9, CYP3A4, Pgp and BCRP; therefore medicines that induce those enzymes may theoretically decrease dolutegravir plasma concentration and reduce the therapeutic effect of DALIDUO. Coadministration of dolutegravir and other medicines that inhibit UGT1A1, UGT1A3, UGT1A9, CYP3A4, and/or Pgp may increase dolutegravir plasma concentration (See table 1 below).
Efavirenz, nevirapine, rifampicin and tipranavir in combination with ritonavir each reduced the plasma concentrations of dolutegravir significantly and require dolutegravir dose adjustment to 50 mg twice daily. Another inducer, fosamprenavir in combination with ritonavir decreased plasma concentrations of dolutegravir but does not require a dosage adjustment of dolutegravir. Caution is warranted and clinical monitoring is recommended when these combinations are given in INI-resistant patients (see table below: Medicines Interactions – HIV 1 Antiviral Medicines). A medicine interaction study with the UGT1A1 inhibitor, atazanavir did not result in a clinically meaningful increase in the plasma concentrations of dolutegravir. Tenofovir, ritonavir, lopinavir/ritonavir, darunavir/ritonavir, rilpivirine, bocepravir, telaprevir, prednisone, rifabutin and omeprazole had no or a minimal effect on dolutegravir pharmacokinetics, therefore no dolutegravir dose adjustment is required when co-administered with these medicines.
There is evidence that the concentration of isoniazid is increased by dolutegravir, as contained in DALIDUO.
Table 1. Medicine Interactions:
Abbreviations ↑ = increase; ↓ = decrease; ↔ = no significant change; AUC = area under the concentration versus time curve; Cmax = maximum observed concentration, CT = concentration at the end of dosing interval.
Rilpivirine
Medicines that affect rilpivirine exposure
Rilpivirine is primarily metabolised by cytochrome P450 (CYP3A), and medicines that induce or inhibit CYP3A may thus affect the clearance of rilpivirine. Coadministration of rilpivirine and medicines that induce CYP3A may result in decreased plasma concentrations of rilpivirine (exposure) which could potentially reduce the therapeutic effect of rilpivirine. Co-administration of rilpivirine and medicines that inhibit CYP3A may result in increased plasma concentrations of rilpivirine. Co-administration of rilpivirine with medicines that increase gastric pH such as PPIs, may result in decreased plasma concentrations of rilpivirine which could potentially reduce the therapeutic effect of DALIDUO.
Medicines that are affected by the use of rilpivirine
QT prolonging medicinal products:
There is limited information available on the potential for a pharmacodynamic interaction between rilpivirine and medicinal products that prolong the QTc interval of the ECG. In a study of healthy subjects, supratherapeutic doses of rilpivirine (75 mg once daily and 300 mg once daily) have been shown to prolong the QTc interval of the ECG (see section 5.1). DALIDUO should be used with caution when co-administered with a medicinal product with a known risk of Torsade de Pointes.
Rilpivirine at a dose of 25 mg (once daily) is not likely to have a clinically relevant effect on the exposure of medicines metabolised by CYP enzymes. Established and theoretical interactions with selected antiretrovirals and non-antiretroviral medicines are listed in Table 1 and Table 2, respectively.
Interaction table:
Interactions between rilpivirine and co-administered medicines are listed in Table 2 and Table 3 below (increase is indicated as ↑, decrease as ↓, no change as ↔, not applicable as NA.
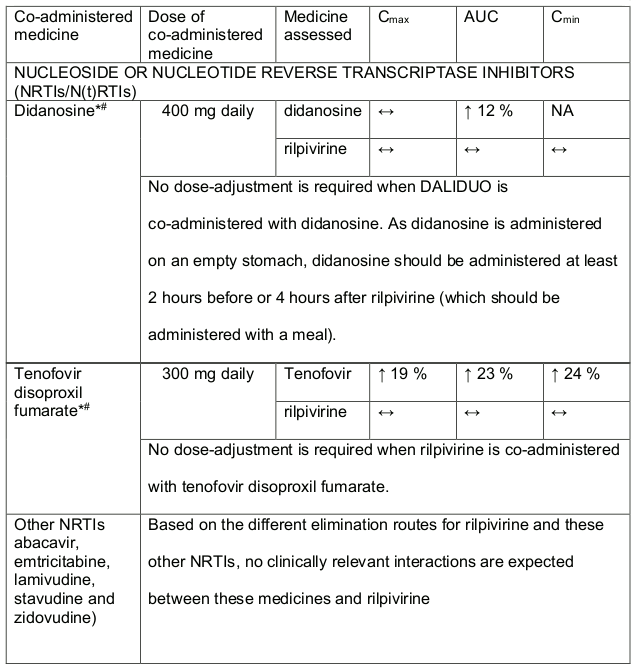
Table 2. Medicine Interactions-Rilpivirine co-administered with antiretroviral and antiviral medicines:
* The Interaction between rilpivirine was evaluated in a clinical study. All other medicine interactions shown may have been predicted.
# This interaction study was performed with a dose higher than the recommended dose for rilpivirine assessing the maximal effect on the co-administered medicine. The dosing recommendation is applicable to the recommended dose of 25 mg rilpivirine daily.
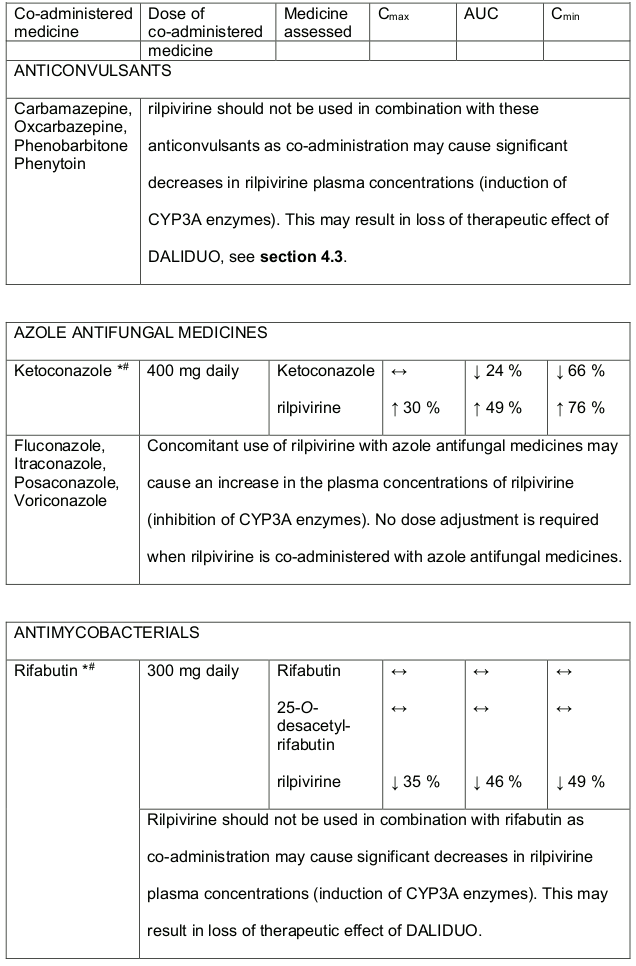
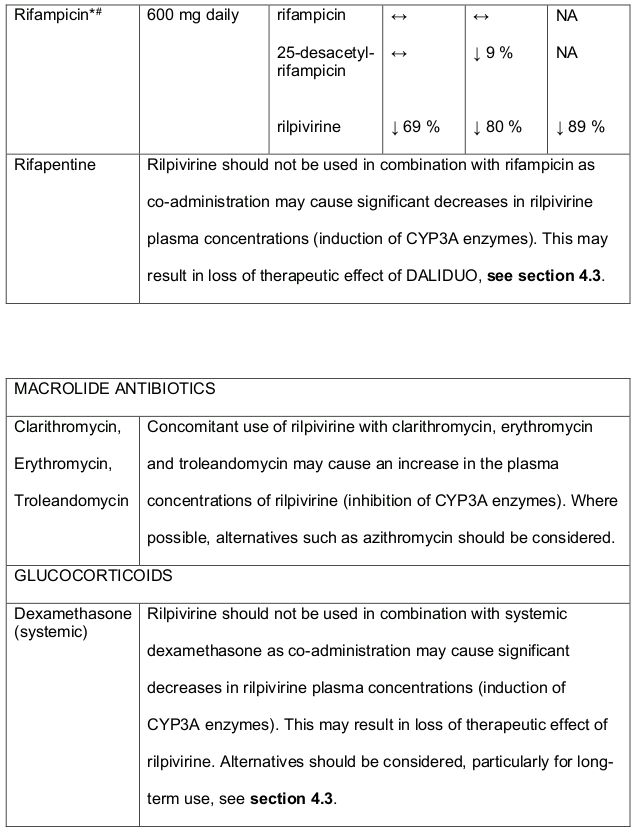
Table 3. Medicine interactions- Rilpivirine co-administered with non-antiretroviral medicines:
* The interaction between rilpivirine and the medicine was evaluated in a clinical study. All other interactions shown are predicted.
# This interaction study has been performed with a dose higher that the recommended dose for rilpivirine assessing the maximal effect on the co-administered medicine. The dosing recommendation is applicable to the recommended dose of rilpivirine 25 mg daily.
1 The interaction between dolutegravir and/or rilpivirine and the medicinal product was evaluated in a clinical study. All other drug-drug interactions shown are predicted.
4.6. Fertility, pregnancy and lactation
Women of child-bearing potential
Adequate contraception Is recommended for women of childbearing potential when taking DALIDUO.
Pregnancy
Safe use during pregnancy has not been established. The effect of dolutegravir on human pregnancy is unknown. In reproductive toxicity studies in animals, dolutegravir was shown to cross the placenta. DALIDUO is contraindicated in peri, preconception period and the first trimester of pregnancy (see section 4.3).
Treatment with DALIDUO should not be started without a medically/laboratory supervised negative pregnancy (urine and/or blood test, repeated at frequent intervals as needed. There is some evidence of neural tube defects with the use of dolutegravir, as contained in DALIDUO, if started at the time of conception or early pregnancy. DALIDUO should not be prescribed to women who plan to become pregnant. Rilpivirine in combination with a background regimen was evaluated in a clinical trial of 19 pregnant women during the second and third trimesters, and post-partum. The pharmacokinetic data demonstrated that total exposure (AUC) rilpivirine as a part of an antiretroviral regimen was approximately 30% lower during pregnancy compared with post-partum (6-12 weeks) Studies in rats and rabbits with rilpivirine have shown no evidence of relevant embryonic or foetal toxicity, effect on reproductive function, or teratogenicity.
Lactation
Safe use during lactation has not been established.
HIV infected women should not breastfeed their infants in order to avoid transmission of HIV. It is expected that dolutegravir will be secreted into human milk. It is not known whether rilpivirine is secreted in human milk. Because of both the potential for HIV transmission and the potential for adverse events in nursing infants, mothers should be instructed not to breastfeed if they are receiving DALIDUO.
Fertility
Animal studies indicate no effect of dolutegravir or rilpivirine on male or female fertility.
4.7. Effects on ability to drive and use machines
DALIDUO may influence the ability to drive and use machines. Dizziness and somnolence may occur. Patients should ensure that they do not engage in driving or operating machines until they know how DALIDUO affects them.
4.8. Undesirable effects
a) Summary of the safety profile
Dolutegravir
Adverse drug reactions (ADRs) identified in an analysis of pooled data from clinical studies are listed below by system organ class and by frequency. Frequencies are defined as: frequent, less frequent or unknown. The safety profile was similar across the treatment naïve, treatment experienced (and integrase naïve) and integrase resistant patient populations.
b) Tabulated (or structured listing) summary of adverse reactions
List of adverse reactions
The following adverse reactions are reported corresponding to: Frequent, Less Frequent and Frequency unknown:
Adverse reactions associated with the fixed dosed combination of dolutegravir and rilpivirine in DALIDUO:
Blood and lymphatic systems disorders
Frequent: decreased white blood cell count, decreased haemoglobin, decreased platelet count
Immune system disorders
Less Frequent: hypersensitivity
Frequency unknown: immune reconstitution syndrome
Metabolism and nutrition disorders
Frequent: increased total cholesterol (fasted), increased LDL cholesterol (fasted), decreased appetite, increased triglycerides (fasted)
Psychiatric disorders
Frequent: insomnia, abnormal dreams, depression, sleep disorders, depressed mood, anxiety
Less Frequent: suicidal ideation or suicide attempt (particularly in patients with a preexisting history of depression or psychiatric illness)
Nervous system disorders
Frequent: headache, dizziness, somnolence
Gastrointestinal disorders
Frequent: nausea, increased pancreatic amylase, diarrhoea, abdominal pain, vomiting, flatulence, increased lipase, abdominal discomfort, upper abdominal pain, dry mouth
Hepatobiliary disorders
Frequent: increased transaminases (alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) elevations), increased bilirubin
Less Frequent: hepatitis, acute hepatic failure**
Skin and subcutaneous tissue disorders
Frequent: rash, pruritus
Musculoskeletal and connective tissue disorders
Less Frequent: arthralgia, myalgia
General disorders and administration site conditions
Frequent: fatigue
Investigations
Frequent: creatine phosphokinase (CPK) elevations
** This adverse reaction was identified through post-marketing surveillance for dolutegravir in combination with other ARVs. The frequency category of rare was estimated based on post-marketing reports.
Adverse reactions associated with dolutegravir, the individual active component of DALIDUO (dolutegravir and rilpivirine):
Immune System Disorders
Less Frequent: hypersensitivity, Immune Reconstitution Syndrome
Psychiatric Disorders
Frequent: Insomnia
Nervous System Disorders
Frequent: headache, dizziness, abnormal dreams
Gastrointestinal Disorders
Frequent: nausea, diarrhoea, vomiting, flatulence, upper abdominal pain
Less Frequent: abdominal pain, abdominal discomfort
Hepatobiliary disorders
Less Frequent: hepatitis
Skin and Subcutaneous Tissue Disorders
Frequent: rash, pruritus
General disorders and administration site conditions
Frequent: fatigue
Adverse reactions associated with rilpivirine, the individual active component of DALIDUO (dolutegravir and rilpivirine):
Blood and lymphatic system disorders
Frequent: increased AST, increased pancreatic amylase
Less Frequent: decreased haemoglobin, decreased platelet count, decreased white
Frequency unknown: increased creatinine
Metabolism and nutrition disorders
Less Frequent: decreased appetite, increased total cholesterol (fasted), increased LDL cholesterol (fasted), increased triglycerides (fasted)
Psychiatric Disorders
Frequent: depression, insomnia,
Less Frequent: abnormal dreams, sleep disorders, depressed mood
Nervous System Disorders
Frequent: headache
Less Frequent: dizziness, somnolence
Gastrointestinal Disorders
Less Frequent: abdominal pain, nausea, vomiting, abdominal discomfort, increased lipase
Hepatobiliary disorders
Less Frequent: increased ALT, increased bilirubin
Skin and Subcutaneous Tissue Disorders
Frequent: rash
General disorders and administration site conditions
Less Frequent: fatigue
Investigations
Frequent: transaminases increased
c) Description of selected adverse reactions
Dolutegravir
Changes in laboratory chemistries
Increases in serum creatinine may have occurred within the first week of treatment with dolutegravir and remained stable through 48 weeks. In treatment naïve patients a mean change from baseline of 9, 96 µmol/L (range: - 53 µmol/L to 54,8 µmol/L) was observed after 48 weeks of treatment. Creatinine increases were comparable by background NRTIs and were similar in treatment experienced patients. These changes are not considered to be clinically relevant since they do not reflect a change in glomerular filtration rate, see section 5.2 – effects on renal function. Small increases in total bilirubin (without clinical jaundice) were observed on dolutegravir and raltegravir (but not efavirenz) arms in the programme. These changes are not considered clinically relevant as they likely reflect competition between dolutegravir and unconjugated bilirubin for a common clearance pathway (UGT1A1). See section 5.2 – metabolism. Asymptomatic creatinine phosphokinase (CPK) elevations mainly in association with exercise have also been reported with dolutegravir therapy.(2: E1)
Rilpivirine
There may be changes from baseline in total cholesterol, LDL- cholesterol, HDL – cholesterol and triglycerides. The impact of such findings has not been demonstrated.
Lipodystrophy
Combination antiretroviral therapy (CART) has been associated with redistribution of body fat (lipodystrophy) in HIV-infected patients, including loss of peripheral and facial subcutaneous fat, increased intra-abdominal and visceral fat, breast hypertrophy and dorsocervical fat accumulation (buffalo hump), see section 4.4. Immune reconstitution syndrome In HIV-infected patients with severe immune deficiency at the time of initiation of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic infections may arise (immune reconstitution syndrome), see section 4.4.
d) Other special populations
Rilpivirine
Patients co-infected with hepatitis B and/or hepatitis C virus
In patients co-infected with hepatitis B or C virus receiving rilpivirine, the incidence of hepatic enzyme elevation was higher than in patients receiving rilpivirine who were not co-infected. The pharmacokinetic exposure of rilpivirine in co-infected patients was comparable to that in patients without co-infection.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicine is important. It allows continued monitoring of the benefit/risk balance of the medicine. Healthcare professionals are asked to report any suspected adverse reactions to SAHPRA via the 6.04 Adverse Drug Reaction Reporting Form, found online under SAHPRA’s publications: https://www.sahpra.org.za/Publications/Index/8 or to Cipla Medpro (Pty) Ltd. (by e-mail: drugsafetysa@cipla.com).
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.