DAYBUE Oral solution Ref.[107361] Active ingredients: Trofinetide
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
The mechanism by which trofinetide exerts therapeutic effects in patients with Rett syndrome is unknown.
12.2. Pharmacodynamics
Cardiac Electrophysiology
At the maximum recommended dose in healthy adult subjects, DAYBUE does not prolong the QT interval to any clinically relevant extent.
12.3. Pharmacokinetics
Trofinetide exhibits linear kinetics with no time- or dose-dependent effect on pharmacokinetic parameters. Systemic exposure to trofinetide was dose-proportional across the studied dose range. Minimal to no accumulation was observed following multiple-dose administration.
Absorption
The time to maximum drug concentration (Tmax) is about 2 to 3 hours after administration. Based on the mass balance study, at least 84% of the administered dose was absorbed following oral administration of 12,000 mg trofinetide.
Effect of Food
Coadministration of DAYBUE with a high-fat meal had no impact on the total exposure (AUC0-inf) of trofinetide and reduced the peak plasma concentration (Cmax) by approximately 20% [see Dosage and Administration (2.2)].
Distribution
Following oral administration, the apparent volume of distribution of trofinetide in adult healthy subjects was approximately 80 L. Trofinetide protein binding in human plasma is less than 6%.
Elimination
The effective elimination half-life of orally administered trofinetide in healthy subjects is about 1.5 hours.
Metabolism
Trofinetide is not significantly metabolized by CYP450 enzymes. Hepatic metabolism is not a significant route of trofinetide elimination.
Excretion
Trofinetide is primarily excreted unchanged (approximately 80% of the dose) in urine, with minor excretion in feces.
Specific Populations
The drug exposure of trofinetide in pediatric patients ages 2 to 4 years of age is similar to children older than 4 years and adults when following the recommended dosage [see Dosage and Administration (2.1)].
The pharmacokinetics in patients with renal impairment have not been studied [see Use in Specific Populations (8.6)].
The pharmacokinetics in patients with hepatic impairment have not been studied. However, hepatic impairment is not expected to impact the exposure of trofinetide because hepatic metabolism is not a significant route of trofinetide elimination.
Drug Interaction Studies
In vitro
Trofinetide is not a substrate of CYP450 enzymes, uridine diphosphate glucuronosyltransferase (UGT), or major drug transporters. Therefore, coadministration of drugs that are inducers or inhibitors of CYP450, UGT, or major drug transporters will not significantly affect the systemic exposure of trofinetide.
Trofinetide is a weak CYP3A4 inhibitor. Using physiologically based pharmacokinetic modeling, coadministration of trofinetide with orally administered midazolam, a sensitive CYP3A4 substrate, was predicted to increase the AUC of midazolam by approximately 1.33-fold [see Drug Interactions (7.1)]. No inhibition on CYP450 enzymes, CYP1A2, 2C8, 2C9, 2C19, and 2D6, is expected at therapeutic systemic concentrations based on the in vitro assays and the static mechanistic models. Time-dependent inhibition on CYP2B6 was inconclusive based on in vitro data. DAYBUE inhibits UGT enzymes, UGT1A9, 2B7, and 2B15, in vitro.
No inhibition was observed at therapeutic systemic concentrations on P-gp, BCRP, BSEP, OAT1, OAT3, OCT2, MATE1, and MATE2-K, based on the in vitro assays. Trofinetide inhibits OATP1B1 and OATP1B3 in vitro [see Drug Interactions (7.1)].
In vivo
There have been no in vivo assessments of drug interactions with trofinetide.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Studies to evaluate the carcinogenic potential of trofinetide have not been conducted.
Mutagenesis
Trofinetide was negative in in vitro (bacterial reverse mutation, chromosomal aberration in Chinese hamster ovary cells) and in vivo (mouse micronucleus) assays.
Impairment of Fertility
Oral administration of trofinetide (0, 150, 450, or 1000 mg/kg twice daily; 0, 300, 900, or 2000 mg/kg/day) to male and female rats prior to and throughout mating and continuing in females through gestation day 7 resulted in no adverse effects on fertility or reproductive function. Plasma exposures at the highest dose tested were less than that in humans at the maximum recommended human dose of 12,000 mg/dose (24,000 mg/day).
14. Clinical Studies
The efficacy of DAYBUE for the treatment of Rett syndrome was established in a 12-week randomized, double-blind, placebo-controlled study in patients with Rett syndrome 5 to 20 years of age (Study 1; NCT04181723).
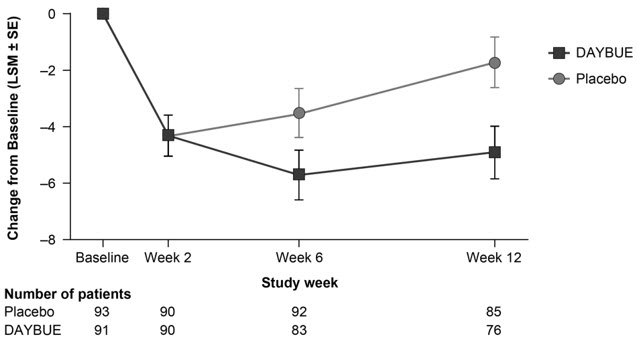
Patients (N=187) had a diagnosis of typical Rett syndrome according to the Rett Syndrome Diagnostic Criteria with a documented disease-causing mutation in the MECP2 gene. Patients were randomized to receive DAYBUE (N=93) or matching placebo (N=94) for 12 weeks. The DAYBUE dosage was based on patient weight to achieve similar exposure in all patients [see Dosage and Administration (2.1)].
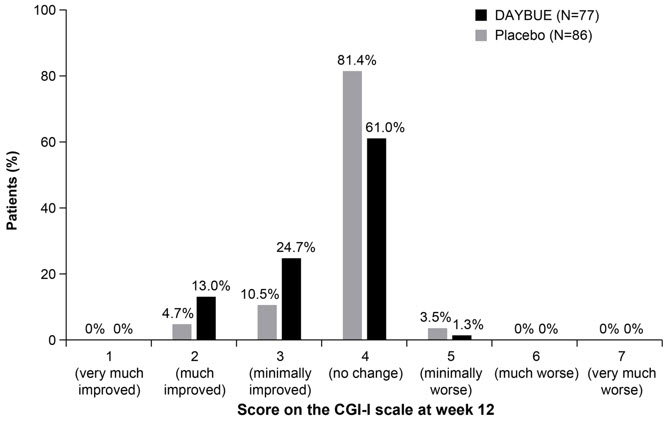
The co-primary efficacy measures were change from baseline after 12 weeks of treatment in the total score of the Rett Syndrome Behaviour Questionnaire (RSBQ) and the Clinical Global Impression-Improvement (CGI-I) score. The RSBQ is a 45-item rating scale completed by the caregiver that assesses a range of symptoms of Rett syndrome (breathing, hand movements or stereotypies, repetitive behaviors, night-time behaviors, vocalizations, facial expressions, eye gaze, and mood). Each item is scored as 0 (not true), 1 (somewhat or sometimes true), or 2 (very true or often true), with a maximum possible score of 90 points. Lower scores reflect lesser severity in signs and symptoms of Rett syndrome. The CGI-I is rated by clinicians to assess whether a patient has improved or worsened on a 7-point scale (1=very much improved to 7=very much worse) in which a decrease in score indicates improvement.
Treatment with DAYBUE demonstrated a statistically significant difference in favor of DAYBUE as compared to placebo on the co-primary efficacy endpoints, the change from baseline in RSBQ total score, and the CGI-I score at week 12 (Table 3, Figure 1, and Figure 2).
Table 3. Summary of Study 1 Efficacy Results:
| Mean Baseline Score (SE) | Mean Week 12 Score (SE) | LS Mean Change from Baseline to Week 12 (SE) | DAYBUE- Placebo Treatment Difference, LS Mean (95% CI)* | p-value | ||
|---|---|---|---|---|---|---|
| RSBQ | DAYBUE | 43.7 (1.21) | 39.9 (1.38) | -4.9 (0.94) | -3.2 (-5.7, -0.6) | 0.018 |
| Placebo | 44.5 (1.26) | 42.8 (1.42) | -1.7 (0.90) | |||
| CGI-I | DAYBUE | -- | 3.5 (0.08) | -- | -0.3 (-0.5, -0.1) | 0.003 |
| Placebo | -- | 3.8 (0.06) | ||||
CI=confidence interval; LS mean=least-squares mean; SE=standard error
* Difference in LS mean from the mixed-effect model for repeated measure analysis
Figure 1. Change From Baseline in RSBQ Total Score in Study 1:
Figure 2. Distribution of CGI-I Scores for Patients Completing Study 1:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.