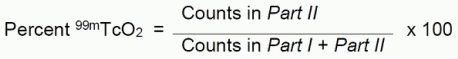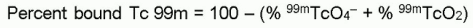DRAXIMAGE DTPA Kit, Powder for solution for injection Ref.[49664] Active ingredients: Technetium ⁹⁹ᵐTc pentetic acid
Source: FDA, National Drug Code (US) Revision Year: 2020
1. Indications and Usage
DRAXIMAGE DTPA, after radiolabeling with Technetium Tc 99m, is indicated for
1.1 Brain Imaging
Brain imaging in adults by intravenous administration.
1.2 Renal Scintigraphy
Renal visualization, assessment of renal perfusion, and estimation of glomerular filtration rate in adult and pediatric patients by intravenous administration.
1.3 Lung Ventilation Imaging
Lung ventilation imaging and evaluation of pulmonary embolism when paired with perfusion imaging in adult and pediatric patients when administered by nebulizer for inhalation.
2. Dosage and Administration
2.1 Radiation Safety – Drug Handling
Tc 99m labeled DRAXIMAGE DTPA injection is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure to the patient and healthcare worker. During preparation and handling, use water proof gloves and effective shielding, including syringe shields [see Warnings and Precautions (5.3)].
2.2 Recommended Dosage and Image Acquisition Instructions
- The recommended dose ranges for intravenous or inhalation administration of DRAXIMAGE DTPA, after reconstitution, are presented in Table 1 through Table 3.
- Do not administer more than one dose.
Table 1. Tc 99m Labeled DRAXIMAGE DTPA Injection – Intravenous Administration, Adults:
| Indication | Route of Administration | Dose | Image Acquisition |
|---|---|---|---|
| Brain Imaging | Intravenous Injection | 370 to 740 MBq (10 to 20 mCi) | Immediate dynamic imaging. Obtain at least one blood-pool image in same position as flow. Delayed images can be obtained 1 hour later. |
| Renal Visualization and Perfusion Assessment | Intravenous Injection | 370 to 740 MBq (10 to 20 mCi) | Immediate dynamic imaging. Static imaging 1 to 30 minutes after injection. |
| Renal Visualization with Estimation of Glomerular Filtration Rate | Intravenous Injection | 111 to 185 MBq (3 to 5 mCi) | Immediate dynamic imaging. Static imaging 1 to 30 minutes after injection. |
| Estimation of Glomerular Filtration Rate (with no renal imaging) | Intravenous Injection | 7.4 to 18.5 MBq (0.2 to 0.5 mCi) | Blood sampling only is performed. |
Table 2. Tc 99m Labeled DRAXIMAGE DTPA Injection – Intravenous Administration, Pediatric Patients:
| Indication | Route of Administration | Dose | Image Acquisition |
|---|---|---|---|
| Renal Visualization and Perfusion Assessment | Intravenous Injection | 3.7 to 7.4 MBq/kg (0.1 to 0.2 mCi/kg) Minimum 37 MBq (1 mCi) Maximum 185 MBq (5 mCi) | Immediate dynamic imaging. Static imaging 1 to 30 minutes after injection. |
| Estimation of Glomerular Filtration Rate (with no renal imaging) | Intravenous Injection | 7.4 to 18.5 MBq (0.2 to 0.5 mCi) | Blood sampling only is performed. |
Table 3. Tc 99m Labeled DRAXIMAGE DTPA – Aerosol Inhalation Administration:
| Indication | Route of Administration | Dose | Image Acquisition |
|---|---|---|---|
| Lung Ventilation Adults | Aerosol Inhalation | 925 to 1850 MBq (25 to 50 mCi) in the nebulizer to achieve a lung dose of approximately 18.5 to 37 MBq (0.5 to 1.0 mCi) | For lung imaging performed prior to perfusion imaging, the target administered dose to the lungs is achieved after 3 to 5 minutes of inhalation or at an imaging count rate of 50,000 to 100,000 per minute*. |
| Lung Ventilation Pediatric Patients | Aerosol Inhalation | 925 MBq (25 mCi) in the nebulizer to achieve a lung dose of approximately 18.5 MBq (0.5 mCi) | For lung imaging performed prior to perfusion imaging, the target administered dose to the lungs is achieved at an imaging count rate of approximately 10,000 to 50,000 per minute*. |
* For lung imaging performed after perfusion imaging, target count rate should be approximately three times that of perfusion count rate.
2.3 Administration Instructions
- Use aseptic technique for all drug preparation and handling.
- Visually inspect the Tc 99m labeled DRAXIMAGE DTPA injection after reconstitution for particulate matter prior to administration. Do not use or administer if there is evidence of foreign matter or the solution is not clear.
- Measure the patient dose by a radioactivity calibration system immediately prior to administration.
Intravenous Use
- Instruct the patient to increase fluid intake and to void frequently for the next 4 to 6 hours after Tc 99m labeled DRAXIMAGE DTPA administration by injection to minimize the radiation dose to the bladder.
Inhalation Use
- Use the selected nebulizer in accordance with the manufacturer’s instructions.
- Instruct the patient to rinse their mouth and expectorate after Tc 99m labeled DRAXIMAGE DTPA administration by inhalation to minimize the radiation dose to the mouth and esophagus.
2.4 Instructions for Drug Preparation
- The prepared solution can either be administered via intravenous injection or aerosolized by nebulizer for inhalation use.
- Before reconstitution, inspect the integrity of the vial.
- Add 2 to 10 mL [maximum amount 18.5 gigabecquerels (500 mCi)] of sodium pertechnetate Tc 99m injection USP to the reaction vial. The volume of pertechnetate added should be balanced by the removal of the same volume of air. Cover the vial shield and invert to mix the contents.
- Assay the preparation in a calibrator, record the radio assay information on the label with radiation warning symbol, and affix it to the reaction vial.
- After reconstitution, store the solution at 25°C (77°F) in a lead shield and discard after 12 hours; excursions permitted between 15 and 30°C (59 and 86°F).
- Allow the preparation to stand for 15 minutes before determining the radiochemical purity of Tc 99m labeled DRAXIMAGE DTPA injection.
- After reconstitution, do not vent the vial.
2.5 Determination of Radiochemical Purity
Obtain the following:
- Two ITLC-SG (1 × 10 cm)
- 0.9% Sodium Chloride Injection USP (for determination of reduced hydrolyzed technetium)
- Acetone (for determination of free pertechnetate)
- Two glass test tubes (18 mm x 150 mm) with stoppers
Step 1:
- System A: Add 1 mL of 0.9% Sodium Chloride Injection USP in an 18 mm x 150 mm test tube. Place the stopper and allow the atmosphere in the tube to equilibrate for 1 minute.
- System B: Repeat with Acetone in a separate test tube.
Step 2:
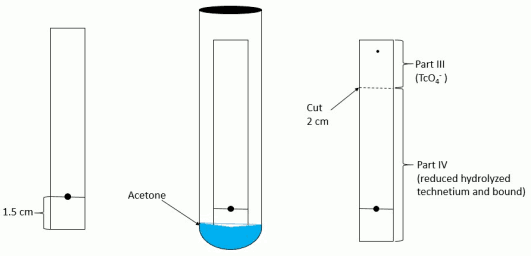
- Mark each chromatographic strip with a pencil mark 1.5 cm (see Figure 1 and Figure 2) from one end of the strip (mark as origin).
- Place one drop (approximately 0.01 – 0.02 mL) of the Technetium Tc 99m pentetate injection at the origin.
- For System A (saline), do not allow the strip to dry.
- For System B (acetone), dry the strip using a gentle stream of nitrogen gas.
Step 3:
- Place each strip with the origin end towards the bottom of the previously equilibrated test tube to develop (the origin must be above the surface of the solvent).
- Place stopper in the test tube and keep upright.
Step 4:
- When the solvent front has reached the top of the strip, remove the strip with forceps and allow it to dry.
Step 5:
System A - Determination of reduced hydrolyzed technetium:
- In System A (saline), reduced hydrolyzed technetium ( 99mTcO2) stays at the origin (Rf 0 to 0.1), while the bound technetium and free pertechnetate ( 99mTcO4 –) migrates to the solvent front (Rf0.85 to 1.0).
- Cut the dried strip 3 cm from the origin.
- The short piece is marked as Part I and the long piece is marked as Part II.
- Count the pieces in a counter and determine the percentage of reduced hydrolyzed technetium according to the following formula:
Figure 1. System A Diagram:
System B - Determination of free pertechnetate:
- In System B (acetone), the bound technetium and reduced hydrolyzed technetium ( 99mTcO2) stay at the origin (Rf 0 to 0.1), while free pertechnetate ( 99mTcO4 –) migrates to the solvent front (Rf 0.85 to 1.0).
- Cut the dried strip 2 cm from the solvent front end.
- The short piece is marked Part III and the long piece is marked Part IV.
- Count the pieces in a counter and determine the percentage of free pertechnetate according to the following formula:
Figure 2. System B Diagram:
Step 6:
- Determine the radiochemical purity according to the following formula:
- Use Technetium Tc 99m pentetate injection only if the radiochemical purity is 90% or greater.
2.6 Radiation Dosimetry
The estimated radiation absorbed dose to various organs from an intravenous injection of Tc 99m pentetate in patients with normal and abnormal renal function is shown respectively in Table 4 and Table 5.
Table 4. Estimated Radiation Absorbed Dose for Technetium Tc 99m Pentetate Injection in Patients With Normal Renal Function Following Intravenous Injection:
| Absorbed Dose Per Unit Activity Administered (μGy/MBq) | |||||
|---|---|---|---|---|---|
| Organ | Adult | 15 Years | 10 Years | 5 Years | 1 Year |
| Adrenals | 1.4 | 1.8 | 2.7 | 4.0 | 7.2 |
| Bone surfaces | 2.4 | 2.9 | 4.3 | 6.1 | 10 |
| Brain | 0.86 | 1.1 | 1.7 | 2.8 | 4.9 |
| Breast | 0.72 | 0.92 | 1.3 | 2.2 | 4.1 |
| Gallbladder wall | 1.5 | 2.1 | 3.8 | 5.0 | 6.1 |
| Gastrointestinal tract | |||||
| Esophagus | 1.0 | 1.3 | 1.9 | 3.0 | 5.4 |
| Stomach wall | 1.3 | 1.7 | 2.8 | 4.0 | 6.8 |
| Small intestine wall | 2.5 | 3.1 | 4.9 | 7.0 | 10 |
| Colon wall | 3.1 | 3.9 | 6.0 | 8.1 | 11 |
| Upper large intestine wall | 2.1 | 2.8 | 4.3 | 6.5 | 9.2 |
| Lower large intestine wall | 4.3 | 5.4 | 8.2 | 10 | 13 |
| Heart wall | 1.2 | 1.5 | 2.2 | 3.3 | 5.9 |
| Kidneys | 4.4 | 5.3 | 7.5 | 11 | 18 |
| Liver | 1.2 | 1.6 | 2.5 | 3.8 | 6.4 |
| Lungs | 1.0 | 1.3 | 2.0 | 3.0 | 5.5 |
| Muscles | 1.6 | 2.0 | 3.0 | 4.3 | 6.8 |
| Ovaries | 4.2 | 5.3 | 7.7 | 10 | 13 |
| Pancreas | 1.4 | 1.8 | 2.8 | 4.3 | 7.4 |
| Red marrow | 1.5 | 1.8 | 2.7 | 3.7 | 5.7 |
| Skin | 0.87 | 1.0 | 1.7 | 2.6 | 4.4 |
| Spleen | 1.3 | 1.6 | 2.6 | 3.9 | 6.8 |
| Testes | 2.9 | 4.0 | 6.8 | 9.4 | 13 |
| Thymus | 1.0 | 1.3 | 1.9 | 3.0 | 5.4 |
| Thyroid | 1.0 | 1.3 | 2.1 | 3.3 | 6.0 |
| Urinary bladder wall | 62 | 78 | 110 | 150 | 170 |
| Uterus | 7.9 | 9.6 | 15 | 18 | 22 |
| Remaining organs | 1.7 | 2.1 | 3.0 | 4.2 | 6.6 |
| Effective dose per unit activity (μSv/MBq) | 4.9 | 6.3 | 9.4 | 12 | 16 |
Table 5. Estimated Radiation Absorbed Dose for Technetium Tc 99m Pentetate Injection in Patients With Abnormal Renal Function Following Intravenous Injection:
| Absorbed Dose Per Unit Activity Administered (μGy/MBq) | |||||
|---|---|---|---|---|---|
| Organ | Adult | 15 Years | 10 Years | 5 Years | 1 Year |
| Adrenals | 4.1 | 5.1 | 7.6 | 11 | 21 |
| Bone surfaces | 6.0 | 7.1 | 11 | 15 | 28 |
| Brain | 2.8 | 3.5 | 5.7 | 9.1 | 16 |
| Breast | 2.3 | 3.0 | 4.2 | 6.8 | 13 |
| Gallbladder wall | 4.2 | 5.7 | 9.2 | 13 | 16 |
| Gastrointestinal tract | |||||
| Esophagus | 3.3 | 4.2 | 6.2 | 9.6 | 17 |
| Stomach wall | 3.8 | 5.0 | 7.9 | 11 | 19 |
| Small intestine wall | 4.5 | 5.6 | 8.5 | 13 | 22 |
| Colon wall | 4.5 | 5.8 | 8.7 | 13 | 22 |
| Upper large intestine wall | 4.3 | 5.6 | 8.1 | 13 | 21 |
| Lower large intestine wall | 4.9 | 6.1 | 9.5 | 13 | 23 |
| Heart wall | 3.7 | 4.7 | 7.0 | 10 | 18 |
| Kidneys | 7.7 | 9.2 | 13 | 19 | 32 |
| Liver | 3.7 | 4.6 | 7.1 | 11 | 19 |
| Lungs | 3.3 | 4.2 | 6.2 | 9.5 | 17 |
| Muscles | 3.2 | 4.0 | 6.1 | 9.1 | 17 |
| Ovaries | 5.0 | 6.2 | 9.2 | 14 | 23 |
| Pancreas | 4.3 | 5.3 | 8.0 | 12 | 21 |
| Red marrow | 3.4 | 4.2 | 6.4 | 9.3 | 16 |
| Skin | 2.2 | 2.6 | 4.2 | 6.7 | 12 |
| Spleen | 3.8 | 4.7 | 7.3 | 11 | 19 |
| Testes | 3.5 | 4.5 | 6.9 | 10 | 18 |
| Thymus | 3.3 | 4.2 | 6.2 | 9.6 | 17 |
| Thyroid | 3.4 | 4.2 | 6.7 | 11 | 19 |
| Urinary bladder wall | 21 | 27 | 39 | 50 | 66 |
| Uterus | 6.1 | 7.4 | 11 | 16 | 25 |
| Remaining organs | 3.3 | 4.1 | 6.3 | 9.7 | 17 |
| Effective dose per unit activity (μSv/MBq) | 4.6 | 5.8 | 8.7 | 13 | 21 |
The estimated radiation absorbed dose to various organs from the inhalation of Tc 99m Pentetate Injection is shown in Table 6.
Table 6. Estimated Radiation Absorbed Dose for Technetium Tc 99m Pentetate Injection Administered by Inhalation*:
| Absorbed Dose Per Unit Activity Administered (μGy/MBq) | |||||
|---|---|---|---|---|---|
| Organ | Adult | 15 Years | 10 Years | 5 Years | 1 Year |
| Adrenals | 2.1 | 2.9 | 4.4 | 6.7 | 12 |
| Bone surfaces | 1.9 | 2.4 | 3.5 | 5.3 | 9.8 |
| Breast | 1.9 | 1.9 | 3.3 | 4.8 | 7.8 |
| Gastrointestinal tract | |||||
| Stomach wall | 1.7 | 2.2 | 3.5 | 5.1 | 8.9 |
| Small intestine wall | 2.1 | 2.6 | 4.1 | 6.3 | 11 |
| Upper large intestine wall | 1.9 | 2.4 | 3.8 | 6.1 | 10 |
| Lower large intestine wall | 3.2 | 4.2 | 6.3 | 8.8 | 15 |
| Kidneys | 4.1 | 5.1 | 7.2 | 11 | 19 |
| Liver | 1.9 | 2.5 | 3.7 | 5.5 | 9.7 |
| Lungs | 17 | 26 | 36 | 54 | 100 |
| Ovaries | 3.3 | 4.1 | 6.1 | 8.9 | 15 |
| Pancreas | 2.1 | 2.6 | 4.0 | 6.1 | 11 |
| Red marrow | 2.7 | 3.4 | 4.7 | 6.2 | 9.6 |
| Spleen | 1.9 | 2.4 | 3.6 | 5.6 | 9.9 |
| Testes | 2.1 | 3.1 | 5.2 | 7.9 | 15 |
| Thyroid | 0.99 | 1.7 | 2.7 | 4.4 | 7.8 |
| Urinary bladder wall | 47 | 58 | 84 | 120 | 230 |
| Uterus | 5.9 | 7.2 | 11 | 16 | 27 |
| Other tissue | 1.8 | 2.2 | 3.2 | 4.9 | 8.6 |
| Effective dose per unit activity (μSv/MBq) | 5.9 | 8.0 | 11 | 17 | 31 |
16.2. Storage and Handling
Store the unreconstituted reaction vials at 25°C (77°F); excursions permitted between 15 and 30°C (59 and 86°F).
This radiopharmaceutical is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.