ELAHERE Concentrate for solution for infusion Ref.[113939] Active ingredients: Mirvetuximab soravtansine
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061 Ludwigshafen, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic and immunomodulating agents, monoclonal antibodies and antibody drug conjugates, other monoclonal antibodies and antibody drug conjugates
ATC code: L01FX26
Mechanism of action
Mirvetuximab soravtansine is an antibody-drug conjugate. The antibody is an engineered IgG1 directed against folate receptor alpha (FRα). The function of the antibody portion is to bind to FRα expressed on the surface of ovarian cancer cells. DM4 is a microtubule inhibitor attached to the antibody via a cleavable linker. Upon binding to FRα, mirvetuximab soravtansine is internalised followed by intracellular release of DM4 via proteolytic cleavage. DM4 disrupts the microtubule network within the cell, resulting in cell cycle arrest and apoptotic cell death.
Pharmacodynamic effects
Cardiac electrophysiology
At the approved recommended dose, mirvetuximab soravtansine did not cause mean increases >10 msec in the QTc interval based on the results of concentration-QTc analysis.
Clinical efficacy and safety
Study IMGN853-0416 (MIRASOL)
The efficacy and safety of mirvetuximab soravtansine were studied in Study IMGN853-0416, a multicentre, open-label, active-controlled, randomised, two-arm phase 3 study that enrolled platinum- resistant advanced high-grade serous epithelial ovarian, primary peritoneal or fallopian tube cancers patients whose tumours (including archival tissue) were FRα positive as determined by the FOLR1 (FOLR1-2.1) RxDx assay (≥75% of viable tumour cells with moderate (2) and/or strong (3) membrane staining intensity by immunohistochemistry (IHC)).
Platinum-resistant disease was defined as EOC that recurred within 6 months of the last dose of platinum.
The study excluded patients with primary platinum-refractory disease, patients with ECOG≥2 and patients with active or chronic corneal disorders, ocular conditions requiring ongoing treatment, Grade ≥2 peripheral neuropathy, or non-infectious ILD/pneumonitis.
Patients were randomised 1:1 to receive either ELAHERE 6 mg/kg AIBW IV (N=227) at Day 1 of each 3-week cycle or one of the following chemotherapies (N=226) as decided by the investigator prior to randomisation:
- Paclitaxel (Pac) 80 mg/m² administered once weekly within a 4-week cycle;
- Pegylated liposomal doxorubicin (PLD) 40 mg/m² administered once every 4 weeks;
- Topotecan (Topo) 4 mg/m² administered on Days 1, 8, and 15 every 4 weeks or for 5 consecutive days at 1.25 mg/m² from Days 1-5 of each 21-day cycle
Randomisation was stratified by number of prior lines of therapy (1 vs 2 vs 3) and by Investigator's choice of chemotherapy (IC Chemo) (Pac vs PLD vs Topo). Treatment was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity.
The primary efficacy outcome measure was progression free survival (PFS) based on investigator assessment using RECIST 1.1 criteria. Objective response rate (ORR) and overall survival (OS) were key secondary efficacy outcome measures.
In total, 453 patients were randomised. The median age was 63 years (range: 29 to 88 years), and patients were predominantly white (66%; 12% Asian). Most patients (80%) had ovarian cancer of epithelial origin; 11% of the fallopian tube; 8% of primary peritoneal; all (100%) were of high-grade serous histology. Approximately half the patients (47%) received 3 prior systemic therapies, 39% had 2 prior lines, and 14% of patients had 1 prior line. The majority of patients received a prior poly ADP ribose polymerase (PARP) inhibitor (55%) and prior bevacizumab (62%). The platinum-free interval following the most recent line of therapy was ≤3 months in 41% of patients, and 3 to 6 months in 58% of patients. Fifty five percent (55%) of patients had an ECOG performance status of 0, and 44% had an ECOG of 1.
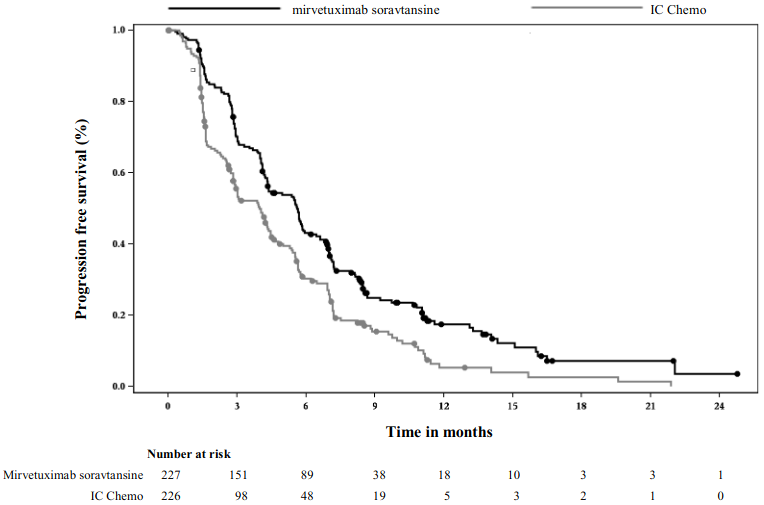
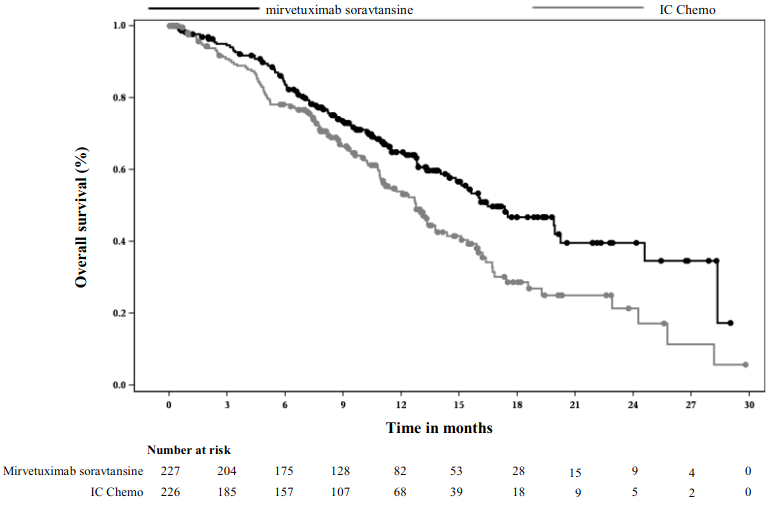
The primary analysis demonstrated a statistically significant improvement in PFS and OS for patients randomised to ELAHERE as compared with IC chemotherapy.
Table 5 summarises the efficacy results of study IMGN853-0416 (MIRASOL).
Table 5. Efficacy results of Study IMGN853-0416:
| Efficacy parameter | ELAHERE N=227 | IC chemotherapies N=226 |
|---|---|---|
| Progression-free survival (PFS) as assessed by investigator | ||
| Number of events (%) | 176 (77.5) | 166 (73.5) |
| Median, months (95% CI) | 5.62 (4.34, 5.95) | 3.98 (2.86, 4.47) |
| Hazard ratio (95% CI) | 0.65 (0.521, 0.808) | |
| p-value | <0.0001 | |
| Overall survival (OS) | ||
| Number of events (%) | 90 (39.6) | 114 (50.4) |
| Median, months (95% CI) | 16.46 (14.46, 24.57) | 12.75 (10.91, 14.36) |
| Hazard ratio (95% CI) | 0.67 (0.504, 0.885) | |
| p-value | 0.0046* | |
Data cut-off 06 March 2023.
* pre-determined efficacy boundary = 0.01313, 2-sided (adjusted by observed number of deaths 204).
The Kaplan Meier curves for investigator-assessed PFS (median follow-up of 11.2 months) and OS (median follow-up of 13.1 months) are presented in Figure 1 and Figure 2.
Figure 1. Kaplan-Meier curve for progression-free survival by treatment arm in MIRASOL (intent to treat population):
Figure 2. Kaplan-Meier curve for overall survival by treatment arm in MIRASOL (intent to treat population):
At an additional descriptive analysis with median follow-up of 20.3 months, OS results were consistent with the primary analysis.
Immunogenicity
Anti-drug antibodies (ADA) were commonly detected. No evidence of ADA impact on pharmacokinetics, efficacy or safety was observed, however, data are still limited.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with ELAHERE in all subsets of the paediatric population in treatment of ovarian carcinoma, treatment of fallopian tube carcinoma, and treatment of peritoneal carcinoma (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
The pharmacokinetics were characterised after patients were administered mirvetuximab soravtansine 0.161 mg/kg to 8.71 mg/kg AIBW doses (i.e., 0.0268 times to 1.45 times the approved recommended dose of 6 mg/kg AIBW), unless otherwise noted.
Table 6 summarises the exposure parameters of mirvetuximab soravtansine, unconjugated DM4, and its metabolite S-methyl-DM4 following administration after the first cycle (3-weeks) of mirvetuximab soravtansine 6 mg/kg to patients. Peak mirvetuximab soravtansine concentrations were observed near the end of intravenous infusion, while peak unconjugated DM4 concentrations were observed on the second day after administration of mirvetuximab soravtansine, and the peak S-methyl-DM4 concentrations were observed approximately 3 days after administration of mirvetuximab soravtansine. Steady state concentrations of mirvetuximab soravtansine, DM4, and S-methyl-DM4 were reached after 1 treatment cycle. Accumulation of the mirvetuximab soravtansine, DM4, and S-methyl-DM4 was minimal following repeat administration of mirvetuximab soravtansine.
Table 6. Exposure parameters of mirvetuximab soravtansine, unconjugated DM4, and S-methyl DM4 after first treatment cycle of 6 mg/kg of mirvetuximab soravtansine:
| Mirvetuximab soravtansine Mean (±SD) | Unconjugated DM4 Mean (±SD) | S-methyl-DM4 Mean (±SD) | |
|---|---|---|---|
| Cmax | 137.3 (±62.3) μg/mL | 4.11 (±2.29) ng/mL | 6.98 (±6.79) ng/mL |
| AUCtau | 20.65 (±6.84) h*mg/mL | 530 (±245) h*ng/mL | 1848 (±1585) h*ng/mL |
Cmax = maximum concentration, AUCtau = area under the concentration vs. time curve over the dosing interval (21 days).
Absorption
Mirvetuximab soravtansine is administered as an intravenous infusion. There have been no studies performed with other routes of administration.
Distribution
The mean (±SD) steady state volume of distribution of mirvetuximab soravtansine was 2.63 (±2.98) L. Human plasma protein binding of DM4 and S-methyl DM4 was >99%, in vitro.
Biotransformation
The monoclonal antibody portion of mirvetuximab soravtansine is expected to be metabolized into small peptides by catabolic pathways. Unconjugated DM4 and S-methyl-DM4 undergo metabolism by CYP3A4. In human plasma, DM4 and S-methyl DM4 were identified as the main circulating metabolites, accounting for approximately 0.4% and 1.4% of mirvetuximab soravtansine AUCs, respectively.
Elimination
The mean (±SD) total plasma clearance of mirvetuximab soravtansine was 18.9 (±9.8) mL/hour. The mean terminal phase half-life of mirvetuximab soravtansine after the first dose was 4.9 days. For the unconjugated DM4, the mean (±SD) total plasma clearance was 14.5 (±4.5) mL/hour and the mean terminal phase half-life was 2.8 days. For S-methyl-DM4, the mean (±SD) total plasma clearance was 5.3 (±3.4) L/hour and the mean terminal phase half-life was 5.1 days. In vitro and nonclinical in vivo studies indicate that DM4 and S-methyl-DM4 are primarily metabolised by CYP3A4 and eliminated via biliary excretion in the faeces.
Special populations
No clinically significant differences in the pharmacokinetics of mirvetuximab soravtansine were observed based on age (32 to 89 years), race (White, Black, or Asian), body weight (36 to 136 kg), mild hepatic impairment (total bilirubin ≤ULN and any AST >ULN or total bilirubin >1 to 1.5 times ULN and any AST), or mild to moderate renal impairment (CLcr ≥30 and <90 mL/min). The pharmacokinetics of mirvetuximab soravtansine in patients with moderate to severe hepatic impairment (total bilirubin >1.5 ULN with any AST) or severe renal impairment (CLcr 15 to 30 mL/min) is unknown.
Drug interaction studies
In vitro studies
Cytochrome P450 (CYP) enzymes: Unconjugated DM4 is a time-dependent inhibitor of CYP3A4. Unconjugated DM4 and S-methyl DM4 are not direct inhibitors of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A. DM4 and S-methyl DM4 are not inducers of CYP1A2, CYP2B6, or CYP3A4.
Transporter systems: Unconjugated DM4 and S-methyl DM4 are substrates of P-gp but are not inhibitors of P-gp.
5.3. Preclinical safety data
Target organs identified with single-dose administration of mirvetuximab soravtansine in cynomolgus monkeys were limited to skin and cellular depletion of the bone marrow and lymphoid tissue. Repeat dosing in cynomolgus monkeys and Dutch-belted rabbits also indicated ophthalmic findings including corneal microcysts, pigmentation, attenuation and degeneration/necrosis of the corneal epithelium. These findings were dose intensity (dose and schedule) dependent with fewer overall findings and recovery of those findings observed in the 3-week dosing schedule (the clinical dosing schedule).
Carcinogenicity studies have not been conducted with mirvetuximab soravtansine or DM4.
DM4 and S-methyl DM4 were not mutagenic in the bacterial reverse mutation (Ames) assay. DM4 and S-methyl DM4 resulted in micronuclei in polychromatic erythrocytes.
No reproductive or developmental animal toxicity studies were conducted with mirvetuximab soravtansine.
Fertility studies have not been conducted with mirvetuximab soravtansine or DM4. There are no data on the effect of ELAHERE on human fertility. However, given the mechanism of action of ELAHERE leads to microtubule disruption and death of rapidly dividing cells, there is the potential for drug-related fertility effects.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.