EMERADE Solution for injection in a pre-filled pen Ref.[27518] Active ingredients: Epinephrine
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: PharmaSwiss Česká republika s.r.o., Jankovcova 1569/2c, 170 00 Prague 7, Czech Republic
4.1. Therapeutic indications
Emerade is indicated for the emergency treatment of severe acute allergic reactions (anaphylaxis) triggered by allergens in foods, medicines, insect stings or bites, and other allergens as well as for exercise-induced or idiopathic anaphylaxis.
4.2. Posology and method of administration
Posology
The effective dose is usually within the range 5-10 micrograms per kg bodyweight but higher doses may be necessary in some cases.
Paediatric population
Use in children: Emerade 500 micrograms is not recommended for use in children.
Children below 15 kg bodyweight
A dosage below 150 micrograms cannot be administered with sufficient accuracy in children weighing less than 15 kg and use is therefore not recommended unless during a life-threatening situation and under medical advice.
Children between 15 kg and 30 kg bodyweight
The usual dose is 150 micrograms.
Children over 30 kg bodyweight
The usual dose is 300 micrograms.
Adolescent patients over 30 kg bodyweight
The dosage recommendations for adult patients should be followed.
Adults
The recommended dose is 300 micrograms for individuals under 60 kg bodyweight. The recommended dose is 300 to 500 micrograms for individuals over 60 kg bodyweight, depending on clinical judgement.
An initial dose should be administered as soon as symptoms of anaphylaxis are recognised. In the absence of clinical improvement or if deterioration occurs, a second injection with an additional Emerade may be administered 5–15 minutes after the first injection. It is recommended that the patients are prescribed two Emerade pens which they should carry at all times.
Method of administration
Emerade is intended for intramuscular administration of adrenaline.
For single use.
Emerade should be administered early, at the first signs of anaphylaxis. A poor outcome from anaphylaxis is associated with late administration of adrenaline.
Emerade must be injected in the outer side of the thigh.
Massaging around the injection area accelerates absorption.
The injection can be administered through clothing.
The patient/carer should be informed that following each use of Emerade:
- They should call for immediate medical assistance, ask for an ambulance and state ‘anaphylaxis’ even if symptoms appear to be improving (see section 4.4).
- Conscious patients should preferably lie flat with feet elevated but sit up if they have breathing difficulties. Unconscious patients should be placed on their side in the recovery position.
- The patient should if possible remain with another person until medical assistance arrives.
For detailed instruction for use, refer to section 6.6.
4.9. Overdose
An overdose, or an accidental intravascular injection of adrenaline, can originate a sudden increase in blood pressure that can cause cerebral haemorrhage. Severe pulmonary oedema caused by peripheral vasoconstriction together with cardiac stimulation can result in death. Severe pulmonary oedema with difficulty in breathing can be treated with fast-acting α-blockers. Life-threatening heart arrhythmias can be treated with β-blocking agents.
6.3. Shelf life
18 months.
6.4. Special precautions for storage
Store in the original outer carton, however while carrying by patient/carer it is acceptable to store in the specially designed case provided. The pen must always be kept in this case to ensure it is protected.
Store below 25°C. Do not freeze.
6.5. Nature and contents of container
Emerade consists of a pre-filled syringe made of glass with a polyisoprene rubber needle plunger in an auto-injector. Emerade is latex free.
Exposed needle length
Emerade 150 micrograms: 16 mm
Emerade 300 micrograms and Emerade 500 micrograms: 23 mm
Package
Emerade has an outer carton as well as a plastic box to store the auto-injector in.
Pack sizes: 1 or 2 pre-filled pens.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
It is very important that the patient receives detailed information on how to use Emerade.
For single use only.
The expiry date is indicated on the label and on the outer carton and Emerade should not be used after this date.
Discard and replace the auto-injector after expiry date.
Check the solution periodically through the inspection window of the unit by lifting the label to make sure the solution is clear and colourless. Discard and replace Emerade if the solution is discoloured or contains particles.
Emerade should always be carried if at risk of anaphylaxis.
Emerade is designed for easy use and has to be considered as a first aid. Emerade should be used for intramuscular injection only on the outer thigh. The injection occurs when the triggering cylinder is gently pressed into the thigh. This can be done through clothing. Emerade has an opening only at the needle end and none at the opposite end.
Method of administration
The instructions for use must be carefully followed in order to avoid accidental injection.
It is recommended that your family members, carers or teachers are also instructed in the correct use of Emerade.
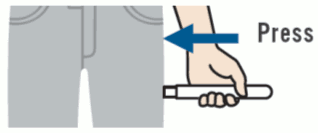
Emerade should only be used for injection in the outer thigh. The injection occurs when Emerade is pressed into the thigh. This can be done through clothing.
1. Remove the needle shield.
2. Place and press Emerade against the outer side of the thigh. A click can be heard when the injection goes into the muscle.
3. Hold Emerade against the thigh for about 5 seconds. Lightly massage the injection site afterwards.
Seek immediate medical help.
The needle in Emerade is protected before, during and after the injection.
When the injection is completed the plunger is visible in the inspection window by lifting the label.
Sometimes a single dose of adrenaline may not be sufficient to completely reverse the effects of a serious allergic reaction. For this reason, your doctor is likely to prescribe more than one Emerade for you. If your symptoms have not improved or have deteriorated within 5-15 minutes after the first injection, either you or the person with you should give a second injection. For this reason you should carry more than one Emerade with you at all times.
Emerade is intended only for emergency treatment. You must always contact your doctor or go to the nearest hospital for further treatment. Inform your doctor that you have taken an injection of adrenaline. Take the used auto-injector with you.
See Section 4.2 for instructions to be conveyed to the patient/carer regarding actions to be taken following each use of Emerade.
Do not remove the needle shield unless injection is required.
Some liquid remains in the auto-injector after the injection. The auto-injector cannot be re-used.
Discard Emerade in accordance with local requirements.
Instructions for use are shown on the label, package and package leaflet.
Autoinjectors without needles are available for training purposes.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.