ENHERTU Powder for concentrate for solution for infusion Ref.[49749] Active ingredients: Trastuzumab deruxtecan
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Daiichi Sankyo Europe GmbH, Zielstattstrasse 48, 81379 Munich, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, HER2 (Human Epidermal Growth Factor Receptor 2) inhibitors
ATC code: L01FD04
Mechanism of action
Enhertu, trastuzumab deruxtecan, is a HER2-targeted antibody-drug conjugate. The antibody is a humanised anti-HER2 IgG1 attached to deruxtecan, a topoisomerase I inhibitor (DXd) bound by a tetrapeptide-based cleavable linker. The antibody-drug conjugate is stable in plasma. The function of the antibody portion is to bind to HER2 expressed on the surface of certain tumour cells. After binding, the trastuzumab deruxtecan complex then undergoes internalisation and intracellular linker cleavage by lysosomal enzymes that are upregulated in cancer cells. Upon release, the membrane-permeable DXd causes DNA damage and apoptotic cell death. DXd, an exatecan derivative, is approximately 10 times more potent than SN-38, the active metabolite of irinotecan.
In vitro studies indicate that the antibody portion of trastuzumab deruxtecan, which has the same amino acid sequence as trastuzumab, also binds to FcγRIIIa and complement C1q. The antibody mediates antibody-dependent cellular cytotoxicity (ADCC) in human breast cancer cells that overexpress HER2. In addition, the antibody inhibits signalling through the phosphatidylinositol 3-kinase (PI3-K) pathway in human breast cancer cells that overexpress HER2.
Clinical efficacy
HER2-positive breast cancer
DESTINY-Breast03 (NCT03529110)
The efficacy and safety of Enhertu were studied in DESTINY-Breast03, a multicentre, open-label, active-controlled, randomised, two-arm phase 3 study that enrolled patients with HER2-positive, unresectable or metastatic breast cancer who received prior trastuzumab and taxane therapy for metastatic disease or developed disease recurrence during or within 6 months of completing adjuvant therapy.
Archival breast tumour samples were required to show HER2 positivity defined as HER2 IHC 3+ or ISH-positive. The study excluded patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening, patients with untreated and symptomatic brain metastases, patients with a history of clinically significant cardiac disease and patients with prior treatment with an anti-HER2 antibody-drug conjugate in the metastatic setting. Patients were randomised 1:1 to receive either Enhertu 5.4 mg/kg (N=261) or trastuzumab emtansine 3.6 mg/kg (N=263) administered by intravenous infusion once every three weeks. Randomisation was stratified by hormone receptor status, prior treatment with pertuzumab, and history of visceral disease. Treatment was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity.
The primary efficacy outcome measure was progression-free survival (PFS) as evaluated by blinded independent central review (BICR) according to Response Evaluation Criteria in Solid Tumours (RECIST v1.1). Overall survival (OS) was a key secondary efficacy outcome measure. PFS based on investigator assessment, confirmed objective response rate (ORR), and duration of response (DOR) were secondary endpoints.
Patient demographics and baseline disease characteristics were balanced between treatment arms. Of the 524 patients randomised, the baseline demographic and disease characteristics were: median age 54 years (range: 20 to 83); 65 years or older (20.2%); female (99.6%); Asian (59.9%), White (27.3%), Black or African American (3.6%); Eastern Cooperative Oncology Group (ECOG) performance status 0 (62.8%) or 1 (36.8%); hormone receptor status (positive: 51.9%); presence of visceral disease (73.3%); presence of brain metastases at baseline (15.6%); and 48.3% of patients received one line of prior systemic therapy in the metastatic setting. The percentage of patients who had not received prior treatment for metastatic disease was 9.5%. The percentage of patients who were previously treated with pertuzumab was 61.1%.
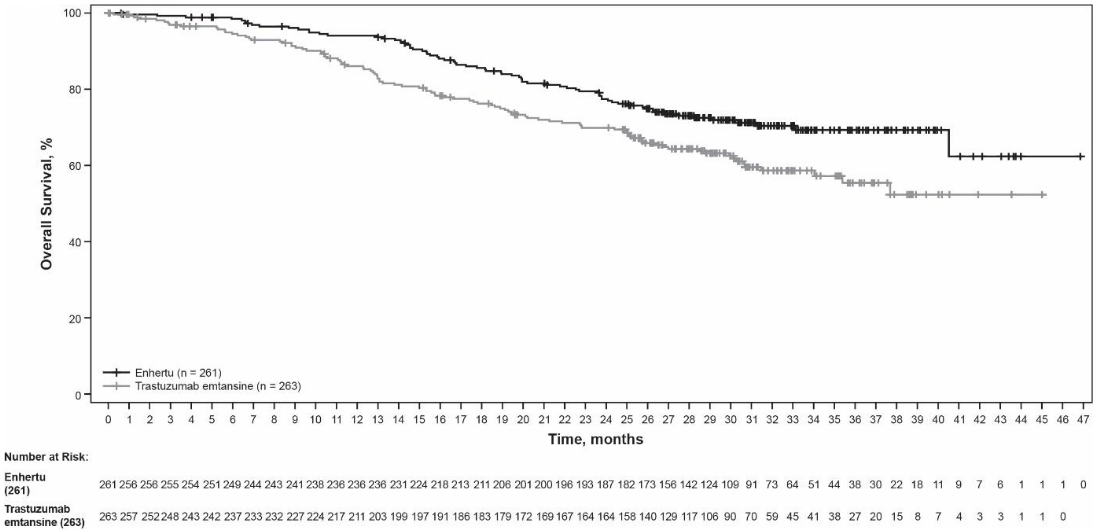
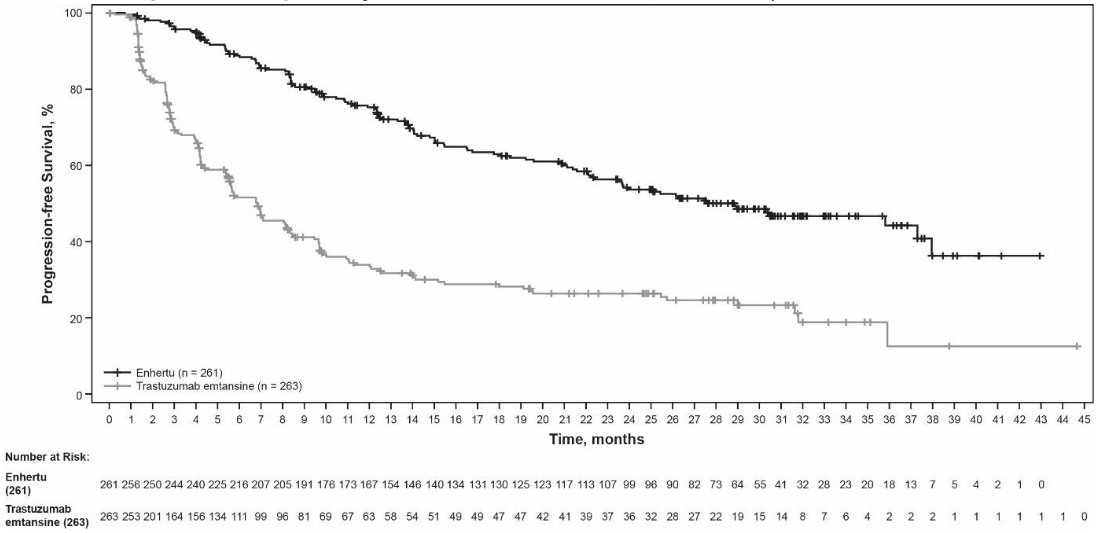
At the prespecified interim analysis for PFS based on 245 events (73% of total events planned for final analysis), the study showed a statistically significant improvement in PFS per BICR in patients randomised to Enhertu compared to trastuzumab emtansine. PFS by BICR data from the primary analysis (data cutoff 21 May 2021) and updated OS, ORR and DOR results from data cutoff 25 July 2022 are presented in Table 4.
Table 4. Efficacy results in DESTINY-Breast03:
| Efficacy parameter | Enhertu N=261 | trastuzumab emtansine N=263 |
|---|---|---|
| Progression-free survival (PFS) per BICRa | ||
| Number of events (%) | 87 (33.3) | 158 (60.1) |
| Median, months (95% CI) | NR (18.5, NE) | 6.8 (5.6, 8.2) |
| Hazard ratio (95% CI) | 0.28 (0.22, 0.37) | |
| p-value | p<0.000001† | |
| Overall survival (OS)b | ||
| Number of events (%) | 72 (27.6) | 97 (36.9) |
| Median, months (95% CI) | NR (40.5, NE) | NR (34.0, NE) |
| Hazard ratio (95% CI) | 0.64 (0.47, 0.87) | |
| p-valuec | p=0.0037 | |
| PFS per BICR (updated)b | ||
| Number of events (%) | 117 (44.8) | 171 (65.0) |
| Median, months (95% CI) | 28.8 (22.4, 37.9) | 6.8 (5.6, 8.2) |
| Hazard ratio (95% CI) | 0.33 (0.26, 0.43) | |
| Confirmed objective response rate (ORR) per BICRb | ||
| n (%) | 205 (78.5) | 92 (35.0) |
| 95% CI | (73.1, 83.4) | (29.2, 41.1) |
| Complete response n (%) | 55 (21.1) | 25 (9.5) |
| Partial response n (%) | 150 (57.5) | 67 (25.5) |
| Duration of response per BICRb | ||
| Median, months (95% CI) | 36.6 (22.4, NE) | 23.8 (12.6, 34.7) |
CI = confidence interval; NE = not estimable; NR = not reached
† presented as 6 decimal places
a Data cutoff 21 May 2021
b Data cutoff 25 July 2022 for a pre-planned OS interim analysis
c The p-value is based on a stratified log-rank test; crossed the efficacy boundary of 0.013.
Figure 1. Kaplan-Meier plot of overall survival (Data cutoff 25 July 2022):
Figure 2. Kaplan-Meier plot of progression-free survival per BICR (Data cutoff 25 July 2022):
Similar PFS results were observed across prespecified subgroups including prior pertuzumab therapy, hormone receptor status, and presence of visceral disease.
DESTINY-Breast02 (NCT03523585)
The efficacy and safety of Enhertu were evaluated in study DESTINY-Breast02, a Phase 3, randomised, multicentre, open-label, active-controlled study that enrolled patients with unresectable or metastatic HER2-positive breast cancer, who were resistant or refractory to prior T-DM1 therapy. Archival breast tumour samples were required to show HER2 positivity defined as HER2 IHC 3+ or ISH-positive. The study excluded patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening, patients with untreated and symptomatic brain metastases and patients with a history of clinically significant cardiac disease. Patients were randomised 2:1 to receive either Enhertu 5.4 mg/kg (n=406) by intravenous infusion every three weeks, or treatment of physician’s choice (n=202, trastuzumab plus capecitabine or lapatinib plus capecitabine). Randomisation was stratified by hormone receptor status, prior treatment with pertuzumab and history of visceral disease. Treatment was administered until disease progression, death, withdrawal of consent or unacceptable toxicity.
The primary efficacy outcome measure was progression-free survival (PFS) as assessed by blinded independent central review (BICR) based on RECIST v1.1. Overall survival (OS) was a key secondary efficacy outcome measure. PFS based on investigator assessment, confirmed objective response rate (ORR) and duration of response (DOR) were secondary objectives.
Demographic and baseline disease characteristics were similar between treatment arms. Of the 608 patients randomised, the median age was 54 years (range 22 to 88); female (99.2%); White (63.2%), Asian (29.3%), Black or African American (2.8%); Eastern Cooperative Oncology Group (ECOG) performance status 0 (57.4%) or 1 (42.4%); hormone receptor status (positive: 58.6%); presence of visceral disease (78.3%); presence of brain metastases at baseline (18.1%) and 4.9% of patients received one line of prior systemic therapy in the metastatic setting.
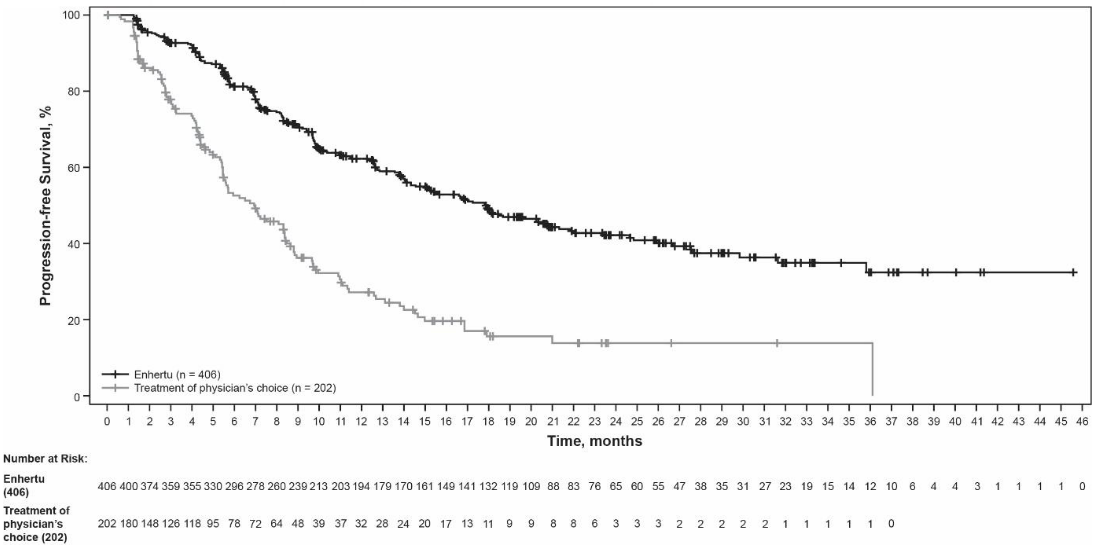
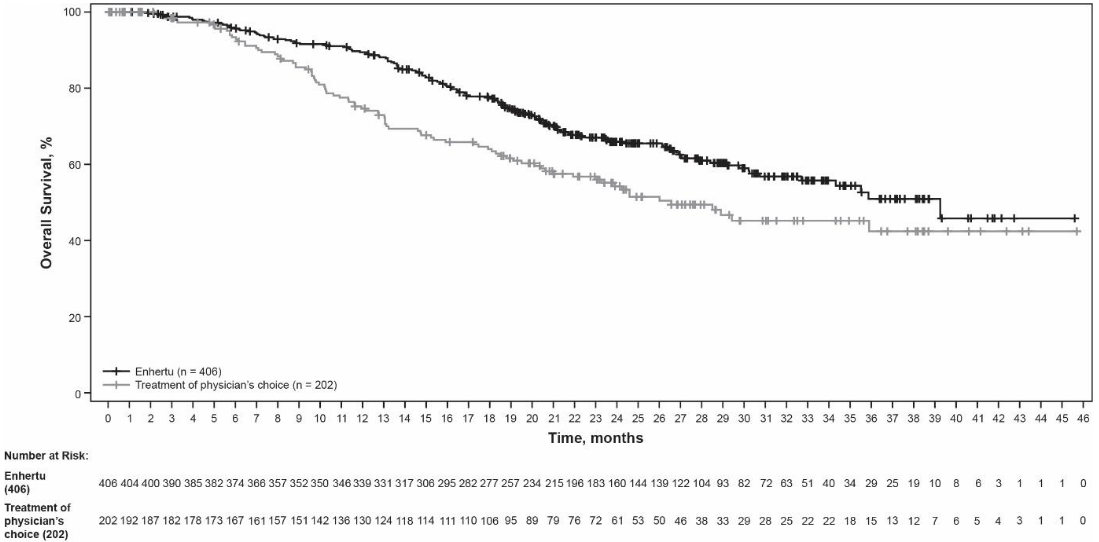
Efficacy results are summarised in Table 5 and Figures 3 and 4.
Table 5. Efficacy results in DESTINY-Breast02:
| Efficacy parameter | Enhertu N=406 | Treatment of physician’s choice N=202 |
|---|---|---|
| PFS per BICR | ||
| Number of events (%) | 200 (49.3) | 125 (61.9) |
| Median, months (95% CI) | 17.8 (14.3, 20.8) | 6.9 (5.5, 8.4) |
| Hazard ratio (95% CI) | 0.36 (0.28, 0.45) | |
| p-value | p<0.000001† | |
| Overall survival (OS) | ||
| Number of events (%) | 143 (35.2) | 86 (42.6) |
| Median, months (95% CI) | 39.2 (32.7, NE) | 26.5 (21.0, NE) |
| Hazard ratio (95% CI) | 0.66 (0.50, 0.86) | |
| p-valuea | p=0.0021 | |
| PFS per investigator assessment | ||
| Number of events (%) | 206 (50.7) | 152 (75.2) |
| Median, months (95% CI) | 16.7 (14.3, 19.6) | 5.5 (4.4, 7.0) |
| Hazard ratio (95% CI) | 0.28 (0.23, 0.35) | |
| Confirmed objective response rate (ORR) per BICR | ||
| n (%) | 283 (69.7) | 59 (29.2) |
| 95% CI | (65.0, 74.1) | (23.0, 36.0) |
| Complete response n (%) | 57 (14.0) | 10 (5.0) |
| Partial response n (%) | 226 (55.7) | 49 (24.3) |
| Duration of response per BICR | ||
| Median, months (95% CI) | 19.6 (15.9, NE) | 8.3 (5.8, 9.5) |
CI = confidence interval; NE = not estimable
† Presented as 6 decimal places
a The p-value is based on a stratified log-rank test; crossed the efficacy boundary of 0.004.
Figure 3. Kaplan-Meier plot of progression-free survival per BICR:
Figure 4. Kaplan-Meier plot of overall survival:
DESTINY-Breast01 (NCT03248492)
The efficacy and safety of Enhertu were studied in DESTINY-Breast01, a multicentre, open-label, single-arm Phase 2 study that enrolled patients with HER2-positive, unresectable and/or metastatic breast cancer who had received two or more prior anti-HER2-based regimens, including trastuzumab emtansine (100%), trastuzumab (100%) and pertuzumab (65.8%). Archival breast tumour samples were required to show HER2 positivity defined as HER2 IHC 3+ or ISH-positive. The study excluded patients with a history of treated ILD or ILD at screening, patients with untreated or symptomatic brain metastases, and patients with a history of clinically significant cardiac disease. Patients enrolled had at least 1 measurable lesion per RECIST v1.1. Enhertu was administered by intravenous infusion at 5.4 mg/kg once every three weeks until disease progression, death, withdrawal of consent, or unacceptable toxicity. The primary efficacy outcome measure was confirmed objective response rate (ORR) according to RECIST v1.1 in the intent-to-treat (ITT) population as evaluated by independent central review (ICR). The secondary efficacy outcome measure was duration of response (DOR).
Of the 184 patients enrolled in DESTINY-Breast01, baseline demographic and disease characteristics were: median age 55 years (range: 28 to 96); 65 years or older (23.9%); female (100%); White (54.9%), Asian (38.0%), Black or African American (2.2%); Eastern Cooperative Oncology Group (ECOG) performance status 0 (55.4%) or 1 (44.0%); hormone receptor status (positive: 52.7%); presence of visceral disease (91.8%); previously treated and stable brain metastases (13.0%); median number of prior therapies in the metastatic setting: 5 (range: 2 to 17); sum of diameters of target lesions (<5 cm: 42.4%, ≥5 cm: 50.0%).
An earlier analysis (median duration of follow-up 11.1 months [range: 0.7 to 19.9 months]) showed a confirmed objective response rate of 60.9% (95% CI: 53.4, 68.0) with 6.0% being complete responders and 54.9% being partial responders; 36.4% had stable disease, 1.6% had progressive disease and 1.1% were not evaluable. Median duration of response at that time was 14.8 months (95% CI: 13.8, 16.9) with 81.3% of responders having a response of ≥6 months (95% CI: 71.9, 87.8). Efficacy results from an updated data cutoff with median duration of follow-up of 20.5 months (range: 0.7 to 31.4 months) are shown in Table 6.
Table 6. Efficacy results in DESTINY-Breast01 (intent-to-treat analysis set):
| DESTINY-Breast01 N=184 | |
|---|---|
| Confirmed objective response rate (95% CI)*† | 61.4% (54.0, 68.5) |
| Complete response (CR) | 6.5% |
| Partial response (PR) | 54.9% |
| Duration of response‡ | |
| Median, months (95% CI) | 20.8 (15.0, NR) |
| % with duration of response ≥6 months (95% CI)§ | 81.5% (72.2, 88.0) |
ORR 95% CI calculated using Clopper-Pearson method
CI = confidence interval
95% CIs calculated using Brookmeyer-Crowley method
* Confirmed responses (by blinded independent central review) were defined as a recorded response of either CR/PR, confirmed by repeat imaging not less than 4 weeks after the visit when the response was first observed.
† Of the 184 patients, 35.9% had stable disease, 1.6% had progressive disease and 1.1% were not evaluable.
‡ Includes 73 patients with censored data.
§ Based on Kaplan-Meier estimation.
NR = not reached
Consistent anti-tumour activity was observed across prespecified subgroups based on prior pertuzumab therapy and hormone receptor status.
HER2-low and HER2-ultralow breast cancer
DESTINY-Breast06 (NCT04494425)
The efficacy and safety of Enhertu were evaluated in study DESTINY-Breast06, a randomised, multicentre, open-label Phase 3 study that randomised 866 adult patients with advanced or metastatic HR+ breast cancer with HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow expression as determined by the PATHWAY/VENTANA anti-HER2/neu (4B5) evaluated at a central laboratory. HER2-ultralow (IHC 0 with membrane staining, described as IHC >0<1+ in the study) is defined as faint and incomplete membrane HER2 staining that is seen in 10% or fewer tumour cells. Patients were eligible if they had disease progression on (a) at least 2 lines of endocrine therapy in the metastatic setting or (b) one line of endocrine therapy in the metastatic setting and demonstrated progression within 24 months of the start of adjuvant endocrine therapy, or within 6 months of starting first line endocrine therapy in combination with a CDK 4/6 inhibitor in the metastatic setting. Patients with prior chemotherapy in the neo-adjuvant or adjuvant setting were eligible if they had a disease-free interval greater than 12 months. The study excluded patients with prior chemotherapy for advanced or metastatic disease, patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening, uncontrolled or significant cardiovascular disease, untreated and symptomatic brain metastases, or ECOG performance status >1.
Patients were randomised 1:1 to receive either Enhertu 5.4 mg/kg (N=436) by intravenous infusion every three weeks or physician’s choice of single agent chemotherapy (N=430, capecitabine 60%, nab-paclitaxel 24%, or paclitaxel 16%). Randomisation was stratified by prior CDK4/6 inhibitor use (yes or no), prior taxane use in the non-metastatic setting (yes or no), and HER2 IHC status of tumour samples (IHC 2+/ISH-, IHC 1+, IHC >0 <1+). Treatment with Enhertu was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity. The primary efficacy outcome measure was PFS in patients with HER2-low breast cancer assessed by BICR based on RECIST v1.1. Key secondary efficacy outcome measures were PFS assessed by BICR based on RECIST v1.1 in the overall population (HER2-low and HER2-ultralow), OS in HER2-low patients, and OS in the overall population. ORR and DOR were secondary endpoints.
In the overall population, demographics and baseline tumour characteristics were similar between treatment arms. Of the 866 patients randomised, the median age was 57 years (range: 28 to 87); 31% were age 65 or older; 99.9% were female; 53% were White, 35% were Asian, and 1% were Black or African American. Patients had an ECOG performance status of 0 (59%) or 1 (39%) at baseline; 18% were IHC >0<1+, 55% were IHC 1+, 27% were IHC 2+/ISH-; 67% had liver metastases, 32% had lung metastases, 8% had brain metastases, and 3% had bone-only metastases. Patients had a median of 2 prior lines of endocrine therapy in the metastatic setting (range: 1 to 5) with 17% having 1 and 68% having 2. Eighty-nine percent of patients had prior endocrine therapy in combination with CDK4/6i treatment in the metastatic setting, 47% had prior anthracycline use, and 41% had prior taxane use in the non-metastatic setting.
Efficacy results are summarised in Table 7 and Figures 5 and 6.
Table 7. Efficacy Results in DESTINY-Breast06:
| Efficacy Parameter | HER2-low | Overall Population (HER2-low and HER2-ultralow) | ||
|---|---|---|---|---|
| Enhertu (N=359) | Chemotherapy (N=354) | Enhertu (N=436) | Chemotherapy (N=430) | |
| Progression Free Survival per BICR | ||||
| Number of events (%) | 225 (62.7) | 232 (65.5) | 269 (61.7) | 271 (63.0) |
| Median, months (95% CI) | 13.2 (11.4, 15.2) | 8.1 (7.0, 9.0) | 13.2 (12.0, 15.2) | 8.1 (7.0, 9.0) |
| Hazard ratio (95% CI) | 0.62 (0.52, 0.75) | 0.64 (0.54, 0.76) | ||
| p-value | <0.0001 | <0.0001 | ||
| Overall Survival* | ||||
| Number of events (%) | 136 (37.9) | 146 (41.2) | 161 (36.9) | 174 (40.5) |
| Median, months (95% CI) | 28.9 (25.7, 33.7) | 27.1 (23.5, 29.9) | 28.9 (26.4, 32.7) | 27.4 (23.9, 29.9) |
| Hazard ratio (95% CI) | 0.83 (0.66, 1.05) | 0.81 (0.66, 1.01) | ||
| Confirmed Objective Response Rate per BICR† | ||||
| n (%) | 203 (56.5) | 114 (32.2) | 250 (57.3) | 134 (31.2) |
| 95% CI | 51.2, 61.7 | 27.4, 37.3 | 52.5, 62.0 | 26.8, 35.8 |
| Duration of Response per BICR† | ||||
| Median, months (95% CI) | 14.1 (11.8, 15.9) | 8.6 (6.7, 11.3) | 14.3 (12.5, 15.9) | 8.6 (6.9, 11.5) |
Data cutoff: 18 March 2024
CI = confidence interval
* First planned interim analysis
† Results were not controlled for type 1 error and should be interpreted descriptively
Consistent PFS benefit was observed across multiple prespecified subgroups, including HER2 expression (IHC >0 <1+, IHC 1+, IHC 2+/ISH-), prior CDK4/6 inhibitor use (yes or no), prior taxane use in the non-metastatic setting (yes or no), and number of prior lines of endocrine therapy in the metastatic setting.
In the HER2-ultralow subgroup (n=152), median PFS was 13.2 months (95% CI: 9.8, 17.3) in patients randomised to Enhertu (N=76) and 8.3 months (95% CI: 5.8, 15.2) in patients randomised to chemotherapy with a hazard ratio of 0.78 (95% CI: 0.50, 1.21). Median OS was 29.5 months (95% CI: 27.9, NE) in patients randomised to Enhertu and 27.4 months (95% CI: 19.4, NE) in patients randomised to chemotherapy with a hazard ratio of 0.75 (95% CI: 0.43, 1.29). Confirmed objective response rate was 61.8% (95% CI: 50.0, 72.8) and 26.3% (95% CI: 16.9, 37.7) in patients randomised to Enhertu and chemotherapy, respectively. Median duration of response was 14.3 months (95% CI: 9.2, 20.7) and 14.1 months (95% CI: 5.9, not estimable) in patients randomised to Enhertu and chemotherapy, respectively.
Figure 5. Kaplan-Meier Plot of Progression Free Survival (Overall Population):
Figure 6. Kaplan-Meier Plot of Overall Survival (Overall Population):
DESTINY-Breast04 (NCT03734029)
The efficacy and safety of Enhertu were studied in DESTINY-Breast04, a phase 3, randomised, multicentre, open-label study that enrolled 557 adult patients with unresectable or metastatic HER2-low breast cancer. The study included 2 cohorts: 494 hormone receptor positive (HR+) patients and 63 hormone receptor negative (HR-) patients. HER2-low expression was defined as IHC 1+ (defined as faint, partial staining of the membrane in greater than 10% of the cancer cells) or IHC 2+/ISH-, as determined by the PATHWAY/VENTANA anti-HER2/neu (4B5) evaluated at a central laboratory. Patients must have received chemotherapy in the metastatic setting or have developed disease recurrence during or within 6 months of completing adjuvant chemotherapy. According to the inclusion criteria, patients who were HR+ must have received at least one endocrine therapy and be ineligible for further endocrine therapy at the time of randomisation. Patients were randomised 2:1 to receive either Enhertu 5.4 mg/kg (N=373) by intravenous infusion every three weeks or physician’s choice of chemotherapy (N=184, eribulin 51.1%, capecitabine 20.1%, gemcitabine 10.3%, nab paclitaxel 10.3%, or paclitaxel 8.2%). Randomisation was stratified by HER2 IHC status of tumour samples (IHC 1+ or IHC 2+/ISH-), number of prior lines of chemotherapy in the metastatic setting (1 or 2) and HR status/prior CDK4/6i treatment (HR+ with prior CDK4/6 inhibitor treatment, HR+ without prior CDK4/6 inhibitor treatment, or HR-). Treatment was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity. The study excluded patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening and clinically significant cardiac disease. Patients were also excluded for untreated or symptomatic brain metastases or ECOG performance status >1.
The primary efficacy endpoint was progression-free survival (PFS) in patients with HR+ breast cancer assessed by BICR based on RECIST v1.1. Key secondary efficacy endpoints were PFS assessed by BICR based on RECIST v1.1 in the overall population (all randomised HR+ and HR- patients), overall survival (OS) in HR+ patients and OS in the overall population. ORR, DOR and patient-reported outcomes (PROs) were secondary endpoints.
Demographics and baseline tumour characteristics were similar between treatment arms. Of the 557 patients randomised, the median age was 57 years (range: 28 to 81); 23.5% were age 65 or older; 99.6% were female and 0.4% were male; 47.9% were White, 40.0% were Asian and 1.8% were Black or African American. Patients had an ECOG performance status of 0 (54.8%) or 1 (45.2%) at baseline; 57.6% were IHC 1+, 42.4% were IHC 2+/ISH-; 88.7% were HR+ and 11.3% HR-; 69.8% had liver metastases, 32.9% had lung metastases, and 5.7% had brain metastases. The percentage of patients who had prior anthracycline use in the (neo)adjuvant setting was 46.3% and 19.4% in the locally advanced and/or metastatic setting. In the metastatic setting, patients had a median of 3 prior lines of systemic therapy (range: 1 to 9) with 57.6% having 1 and 40.9% having 2 prior chemotherapy regimens; 3.9% were early progressors (progression in the neo/adjuvant setting). In HR+ patients, the median number of prior lines of endocrine therapy was 2 (range: 0 to 9) and 70% had prior CDK4/6 inhibitor treatment.
Efficacy results are summarised in Table 8 and Figures 7 and 8.
Table 8. Efficacy results in DESTINY-Breast04:
| Efficacy parameter | HR+ cohort | Overall population (HR+ and HR- cohort) | ||
|---|---|---|---|---|
| Enhertu (N=331) | Chemotherapy (N=163) | Enhertu (N=373) | Chemotherapy (N=184) | |
| Overall survival | ||||
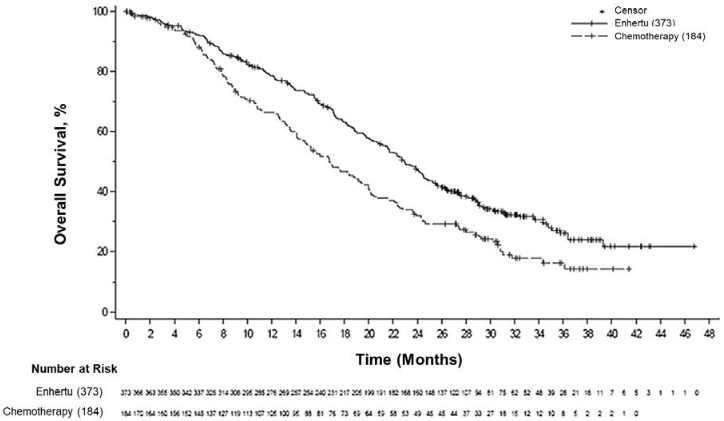
| Number of events (%) | 126 (38.1) | 73 (44.8) | 149 (39.9) | 90 (48.9) |
| Median, months (95% CI) | 23.9 (20.8, 24.8) | 17.5 (15.2, 22.4) | 23.4 (20.0, 24.8) | 16.8 (14.5, 20.0) |
| Hazard ratio (95% CI) | 0.64 (0.48, 0.86) | 0.64 (0.49, 0.84) | ||
| p-value | 0.0028 | 0.001 | ||
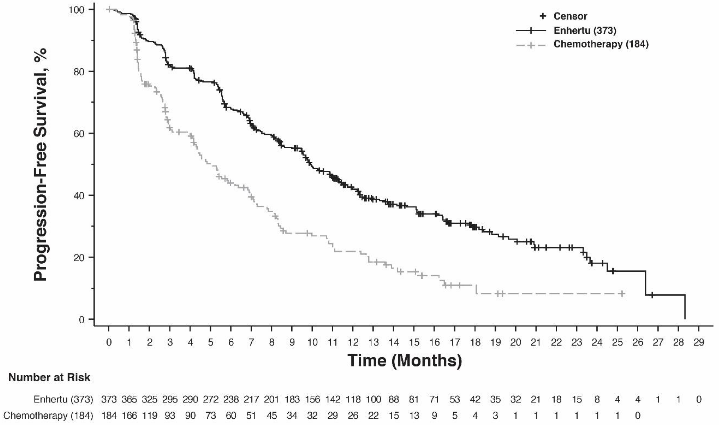
| Progression-free survival per BICR | ||||
| Number of events (%) | 211 (63.7) | 110 (67.5) | 243 (65.1) | 127 (69.0) |
| Median, months (95% CI) | 10.1 (9.5, 11.5) | 5.4 (4.4, 7.1) | 9.9 (9.0, 11.3) | 5.1 (4.2, 6.8) |
| Hazard ratio (95% CI) | 0.51 (0.40, 0.64) | 0.50 (0.40, 0.63) | ||
| p-value | <0.0001 | <0.0001 | ||
| Confirmed objective response rate per BICR* | ||||
| n (%) | 175 (52.6) | 27 (16.3) | 195 (52.3) | 30 (16.3) |
| 95% CI | 47.0, 58.0 | 11.0, 22.8 | 47.1, 57.4 | 11.3, 22.5 |
| Complete Response n (%) | 12 (3.6) | 1 (0.6) | 13 (3.5) | 2 (1.1) |
| Partial Response n (%) | 164 (49.2) | 26 (15.7) | 183 (49.1) | 28 (15.2) |
| Duration of response per BICR* | ||||
| Median, months (95% CI) | 10.7 (8.5, 13.7) | 6.8 (6.5, 9.9) | 10.7 (8.5, 13.2) | 6.8 (6.0, 9.9) |
CI = confidence interval
* Based on data from electronic case report form for the HR+ cohort: N=333 for Enhertu arm and N=166 chemotherapy arm.
Consistent OS and PFS benefit were observed across prespecified subgroups, including HR status, prior CDK4/6i treatment, number of prior chemotherapies and IHC 1+ and IHC 2+/ISH-status. In the HR-subgroup, median OS was 18.2 months (95% CI: 13.6, not estimable) in patients randomised to Enhertu compared to 8.3 months (95% CI: 5.6, 20.6) in patients randomised to chemotherapy with a hazard ratio of 0.48 (95% CI: 0.24, 0.95). Median PFS was 8.5 months (95% CI: 4.3, 11.7) in patients randomised to Enhertu and 2.9 months (95% CI: 1.4, 5.1) in patients randomised to chemotherapy with a hazard ratio of 0.46 (95% CI: 0.24, 0.89).
At an updated descriptive analysis with a median follow-up of 32 months, OS improvements were consistent with the primary analysis. The HR in the overall population was 0.69 (95% CI: 0.55, 0.86) with a median OS of 22.9 months (95% CI: 21.2, 24.5) in the Enhertu arm versus 16.8 months (95% CI: 14.1, 19.5) in the chemotherapy arm. The Kaplan-Meier curve for the updated OS analysis is shown in Figure 7.
Figure 7. Kaplan-Meier plot of overall survival (overall population) (updated analysis):
Figure 8. Kaplan-Meier plot of progression-free survival per BICR (overall population):
NSCLC
DESTINY-Lung02 (NCT04644237)
The efficacy and safety of Enhertu were studied in DESTINY-Lung02, a phase 2, randomised study evaluating two dose levels. The treatment dosage assignment was blinded to patients and investigators. The study included adult patients with metastatic HER2-mutant NSCLC who had received at least one regimen containing platinum-based chemotherapy. Identification of an activating HER2 (ERBB2) mutation was prospectively determined in tumour tissue by local laboratories using a validated test such as next generation sequencing, polymerase chain reaction or mass spectrometry. Patients were randomised 2:1 to receive Enhertu 5.4 mg/kg or 6.4 mg/kg every 3 weeks, respectively. Randomisation was stratified by prior anti-programmed cell death receptor-1 (PD-1) and/or anti-programmed cell death ligand 1 (PD-L1) treatment (yes versus no). Treatment was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity. The study excluded patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening and clinically significant cardiac disease. Patients were also excluded for untreated and symptomatic brain metastases or ECOG performance status >1.
The primary efficacy outcome measure was confirmed ORR as assessed by BICR using RECIST v1.1. The secondary efficacy outcome measure was DOR.
Demographic and baseline disease characteristics from the 102 patients enrolled in the 5.4 mg/kg arm were: median age 59.4 years (range 31 to 84); female (63.7%); Asian (63.7%), White (22.5%), or Other (13.7%); ECOG performance status 0 (28.4%) or 1 (71.6%); 97.1% had a mutation in the ERBB2 kinase domain, 2.9% in the extracellular domain; 96.1% had a HER2 mutation in exon 19 or exon 20; 34.3% had stable brain metastases; 46.1% were former smokers, none were current smokers; 21.6% had a prior lung resection. In the metastatic setting, 32.4% had greater than 2 prior systemic therapies, 100% received platinum-based therapy, 73.5% received anti-PD-1/PD-L1 therapy, and 50.0% had prior treatment with platinum therapy and anti-PD-1/PD-L1 therapy in combination.
Efficacy results are summarised in Table 9. The median duration of follow-up was 11.5 months (data cutoff: 23 December 2022).
Table 9. Efficacy results in DESTINY-Lung02:
| Efficacy parameter | DESTINY-Lung02 5.4 mg/kg N=102 |
|---|---|
| Confirmed objective response rate (ORR) per BICR | |
| n (%) | 50 (49.0) |
| (95% CI)* | (39.0, 59.1) |
| Complete response (CR) n (%) | 1 (1.0) |
| Partial response (PR) n (%) | 49 (48.0) |
| Duration of response | |
| Median, months (95% CI)† | 16.8 (6.4, NE) |
* 95% CI calculated using Clopper-Pearson method
CI = confidence interval, NE = not estimable
† 95% CI calculated using Brookmeyer-Crowley method
Gastric cancer
DESTINY-Gastric02 (NCT04014075)
The efficacy and safety of Enhertu were studied in DESTINY-Gastric02, a Phase 2, multicentre, open- label, single-arm study conducted at sites in Europe and the United States. The study enrolled patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma who had progressed on a prior trastuzumab-based regimen. Patients were required to have centrally confirmed HER2 positivity defined as IHC 3+ or IHC 2+/ISH-positive. The study excluded patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening, patients with a history of clinically significant cardiac disease, and patients with active brain metastases. Enhertu was administered by intravenous infusion at 6.4 mg/kg every three weeks until disease progression, death, withdrawal of consent, or unacceptable toxicity. The primary efficacy outcome measure was confirmed ORR assessed by ICR based on RECIST v1.1. DOR and OS were secondary endpoints.
Of the 79 patients enrolled in DESTINY-Gastric02, demographic and baseline disease characteristics were: median age 61 years (range 20 to 78); 72% were male; 87% were White, 5.0% were Asian and 1.0% were Black or African American. Patients had an ECOG performance status of either 0 (37%) or 1 (63%); 34% had gastric adenocarcinoma and 66% had GEJ adenocarcinoma; 86% were IHC 3+ and 13% were IHC 2+/ISH-positive, and 63% had liver metastases.
Efficacy results for ORR and DOR are summarised in Table 10.
Table 10. Efficacy results in DESTINY-Gastric02 (full analysis set*):
| Efficacy parameter | DESTINY-Gastric02 N=79 |
|---|---|
| Data cutoff date 08 November 2021 | |
| Confirmed objective response rate† % (95% CI)‡ | 41.8 (30.8, 53.4) |
| Complete response n (%) | 4 (5.1) |
| Partial response n (%) | 29 (36.7) |
| Duration of response Median§, months (95% CI)¶ | 8.1 (5.9, NE) |
NE = Not estimable
* Includes all patients who received at least one dose of Enhertu
† Assessed by independent central review
‡ Calculated using Clopper-Pearson method
§ Based on Kaplan-Meier estimate
¶ Calculated using the Brookmeyer and Crowley method
DESTINY-Gastric01 (NCT03329690)
The efficacy and safety of Enhertu were studied in DESTINY-Gastric01, a Phase 2, multicentre, open- label, randomised study conducted at sites in Japan and South Korea. This supportive study included adult patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma who had progressed on at least two prior regimens, including trastuzumab, a fluoropyrimidine agent, and a platinum agent. Patients were randomised 2:1 to receive either Enhertu (N=126) or physician’s choice of chemotherapy: either irinotecan (N=55) or paclitaxel (N=7). Tumour samples were required to have centrally confirmed HER2 positivity defined as IHC 3+ or IHC 2+/ISH-positive. The study excluded patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening, patients with a history of clinically significant cardiac disease, and patients with active brain metastases. Treatment was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity. The primary efficacy outcome measure was unconfirmed ORR assessed by ICR based on RECIST v1.1. Overall survival (OS), progression-free survival (PFS), DOR, and confirmed ORR were secondary outcome measures.
Demographic and baseline disease characteristics were similar between treatment arms. Of the 188 patients, the median age was 66 years (range 28 to 82); 76% were male; 100% were Asian. Patients had an ECOG performance status of either 0 (49%) or 1 (51%); 87% had gastric adenocarcinoma and 13% had GEJ adenocarcinoma; 76% were IHC 3+ and 23% were IHC 2+/ISH-positive; 54% had liver metastases; 29% had lung metastases; the sum of diameters of target lesions was <5 cm in 47%, ≥5 to <10 cm in 30%, and ≥10 cm in 17%; 55% had two and 45% had three or more prior regimens in the locally advanced or metastatic setting.
Efficacy results (data cutoff date: 03 June 2020) for Enhertu (n=126) vs. physician’s choice of chemotherapy (n=62) were confirmed ORR 39.7% (95% CI: 31.1, 48.8) vs. 11.3% (95% CI: 4.7, 21.9). Complete response rate was 7.9% vs. 0% and partial response rate was 31.7% vs. 11.3%. Additional efficacy results for Enhertu vs. physician’s choice of chemotherapy were median DOR of 12.5 months (95% CI: 5.6, NE) vs. 3.9 months (95% CI: 3.0, 4.9). Median PFS was 5.6 months (95% CI: 4.3, 6.9) vs. 3.5 months (95% CI: 2.0, 4.3; hazard ratio = 0.47 [95% CI: 0.31, 0.71]). An OS analysis, prespecified at 133 deaths, showed survival benefit with Enhertu treatment compared to the 25 physician’s choice group (hazard ratio = 0.60). The median OS was 12.5 months (95% CI: 10.3, 15.2) in the Enhertu group and 8.9 months (95% CI: 6.4, 10.4) in the physician’s choice group.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies in all subsets of the paediatric population in breast cancer, NSCLC and gastric cancer (see section 4.2 for information on paediatric use).
This medicinal product has been authorised under a so-called ‘conditional approval’ scheme. This means that further evidence on this medicinal product is awaited. The European Medicines Agency will review new information on this medicinal product at least every year and this SmPC will be updated as necessary.
5.2. Pharmacokinetic properties
Absorption
Trastuzumab deruxtecan is administered intravenously. There have been no studies performed with other routes of administration.
Distribution
Based on population pharmacokinetic analysis, the volume of distribution of the central compartment (Vc) of trastuzumab deruxtecan and topoisomerase I inhibitor, DXd, were estimated to be 2.68 L and 28.0 L, respectively.
In vitro, the mean human plasma protein binding of DXd was approximately 97%.
In vitro, the blood to plasma concentration ratio of DXd was approximately 0.6.
Biotransformation
Trastuzumab deruxtecan undergoes intracellular cleavage by lysosomal enzymes to release the DXd.
The humanised HER2 IgG1 monoclonal antibody is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
In vitro metabolism studies in human liver microsomes indicate that DXd is metabolised mainly by CYP3A4 via oxidative pathways.
Elimination
Following intravenous administration of trastuzumab deruxtecan in patients with metastatic HER2-positive, HER2-low breast cancer or HER2-mutant NSCLC, the clearance of trastuzumab deruxtecan in population pharmacokinetic analysis was calculated to be 0.4 L/day and the clearance of DXd was 18.4 L/h. In patients with locally advanced or metastatic gastric or GEJ adenocarcinoma, trastuzumab deruxtecan clearance was 20% higher than in patients with metastatic HER2-positive breast cancer. In cycle 3, the apparent elimination half-life (t1/2) of trastuzumab deruxtecan and released DXd was approximately 7 days. Moderate accumulation (approximately 35% in cycle 3 compared to cycle 1) of trastuzumab deruxtecan was observed.
Following intravenous administration of DXd to rats, the major excretion pathway was faeces via the biliary route. DXd was the most abundant component in urine, faeces, and bile. Following single intravenous administration of trastuzumab deruxtecan (6.4 mg/kg) to monkeys, unchanged released DXd was the most abundant component in urine and faeces. DXd excretion was not studied in humans.
In vitro interactions
Effects of Enhertu on the pharmacokinetics of other medicinal products
In vitro studies indicate DXd does not inhibit major CYP450 enzymes including CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A. In vitro studies indicate that DXd does not inhibit OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, MATE2-K, P-gp, BCRP, or BSEP transporters.
Effects of other medicinal products on the pharmacokinetics of Enhertu
In vitro, DXd was a substrate of P-gp, OATP1B1, OATP1B3, MATE2-K, MRP1, and BCRP. No clinically meaningful interaction is expected with medicinal products that are inhibitors of MATE2-K, MRP1, P-gp, OATP1B, or BCRP transporters (see section 4.5).
Linearity/non-linearity
The exposure of trastuzumab deruxtecan and released DXd when administered intravenously increased in proportion to dose in the 3.2 mg/kg to 8.0 mg/kg dose range (approximately 0.6 to 1.5 times the recommended dose) with low to moderate inter-subject variability. Based on population pharmacokinetic analysis, inter-subject variability in trastuzumab deruxtecan and DXd elimination clearances were 24% and 28%, respectively, and for central volume of distribution were 16% and 55%, respectively. The intra-subject variability in trastuzumab deruxtecan and DXd AUC values (area under the serum concentration versus time curve) was approximately 8% and 14%, respectively.
Special populations
Based on population pharmacokinetic analysis, age (20-96 years), race, ethnicity, sex and body weight did not have a clinically meaningful effect on exposure of trastuzumab deruxtecan or released DXd.
Elderly
The population PK analysis showed that age (range: 20-96 years) did not affect the PK of trastuzumab deruxtecan.
Renal impairment
No dedicated renal impairment study was conducted. Based on population pharmacokinetic analysis including patients with mild (creatinine clearance [CLcr] ≥60 and <90 mL/min) or moderate (CLcr ≥30 and <60 mL/min) renal impairment (estimated by Cockcroft-Gault), the pharmacokinetics of the released DXd was not affected by mild or moderate renal impairment as compared to normal renal function (CLcr ≥90 mL/min).
Hepatic impairment
No dedicated hepatic impairment study was conducted. Based on population pharmacokinetic analysis, the impact of changes on pharmacokinetics of trastuzumab deruxtecan in patients with total bilirubin ≤1.5 times ULN, irrespective of AST level, is not clinically meaningful. There is limited data for patients with total bilirubin >1.5 to 3 times ULN, irrespective of AST level, to draw conclusions, and no data is available for patients with total bilirubin >3 times ULN, irrespective of AST level (see sections 4.2 and 4.4).
Paediatric population
No studies have been conducted to investigate the pharmacokinetics of trastuzumab deruxtecan in children or adolescents.
5.3. Preclinical safety data
In animals, toxicities were observed in lymphatic and haematopoietic organs, intestines, kidneys, lungs, testes and skin following the administration of trastuzumab deruxtecan at exposure levels of the topoisomerase I inhibitor (DXd) below clinical plasma exposure. In these animals, antibody-drug conjugate (ADC) exposure levels were similar or above clinical plasma exposure.
DXd was clastogenic in both an in vivo rat bone marrow micronucleus assay and an in vitro Chinese hamster lung chromosome aberration assay and was not mutagenic in an in vitro bacterial reverse mutation assay.
Carcinogenicity studies have not been conducted with trastuzumab deruxtecan.
Dedicated fertility studies have not been conducted with trastuzumab deruxtecan. Based on results from general animal toxicity studies, trastuzumab deruxtecan may impair male reproductive function and fertility.
There were no animal reproductive or developmental toxicity studies conducted with trastuzumab deruxtecan. Based on results from general animal toxicity studies, trastuzumab deruxtecan and DXd were toxic to rapidly dividing cells (lymphatic/haematopoietic organs, intestine, or testes), and DXd was genotoxic, suggesting the potential for embryotoxicity and teratogenicity.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.