FABHALTA Hard capsule Ref.[107283] Active ingredients: Iptacopan
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
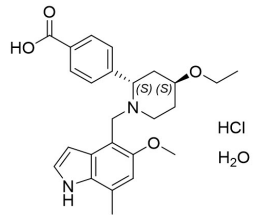
FABHALTA contains iptacopan, a complement Factor B inhibitor. The molecular weight of iptacopan hydrochloride monohydrate is approximately 477 g/mol. The chemical name is (2S,4S)2(4-Carboxyphenyl)-4-ethoxy-1-[(5-methoxy-7-methyl-1H-indol-4-yl)methyl]piperidin-1-ium chloride―water (1/1). The molecular formula is C25H30N2O4·HCl H2O. The structure is shown below.
Iptacopan hydrochloride monohydrate is a white or almost white to pale purplish-pink powder.
FABHALTA is supplied as hard gelatin capsules for oral administration. The capsules are packaged in high-density polyethylene (HDPE) bottles with induction seals and child resistant caps. Each FABHALTA capsule contains 200 mg iptacopan (provided as 225.8 mg iptacopan hydrochloride monohydrate) and the capsule shell contains the following inactive ingredients: gelatin, red ferric oxide, titanium dioxide, yellow ferric oxide. The black printing ink contains ferrosoferric oxide, potassium hydroxide, propylene glycol, shellac, and strong ammonia solution.
| Dosage Forms and Strengths |
|---|
|
Capsules: 200 mg of iptacopan in pale yellow, opaque, hard gelatin capsules imprinted with “LNP200” on the body and “NVR” on the cap. |
| How Supplied |
|---|
|
200 mg capsules: pale yellow opaque hard capsules, imprinted with “LNP200” on one half and “NVR” on the other half, packaged in a high-density polyethylene (HDPE) bottle with induction seal and child-resistant cap. Each bottle contains 60 capsules (NDC 0078-1189-20). Distributed by: Novartis Pharmaceuticals Corporation, East Hanover, New Jersey 07936 |
Drugs
| Drug | Countries | |
|---|---|---|
| FABHALTA | Austria, France, Lithuania, Romania, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.