FABHALTA Hard capsule Ref.[110453] Active ingredients: Iptacopan
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, complement inhibitors
ATC code: L04AJ08
Mechanism of action
Iptacopan is a proximal complement inhibitor that targets Factor B (FB) to selectively inhibit the alternative pathway. Inhibition of FB in the alternative pathway of the complement cascade prevents the activation of C3 convertase and the subsequent formation of C5 convertase to control both C3-mediated extravascular haemolysis (EVH) and terminal complement-mediated intravascular haemolysis (IVH).
Pharmacodynamic effects
The onset of inhibition of the alternative complement pathway, measured using an ex vivo alternative pathway assay, Bb levels (fragment b of Factor B) and plasma levels of C5b-9, was ≤2 hours after a single iptacopan dose in healthy volunteers.
A comparable effect of iptacopan was observed in patients with PNH previously exposed to anti-C5 agents and treatment-naïve patients.
In treatment-naïve PNH patients, iptacopan 200 mg twice daily reduced LDH by >60% compared to baseline after 12 weeks and maintained the effect through to the end of the study.
Cardiac electrophysiology
In a QTc clinical study in healthy volunteers, single supra-therapeutic iptacopan doses up to 1 200 mg (which provided greater than 4-fold exposure of the 200 mg twice daily dose), showed no effect on cardiac repolarisation or QT interval.
Clinical efficacy and safety
The efficacy and safety of iptacopan in adult patients with PNH were evaluated in two multicentre, open-label, 24-week phase III studies: an active comparator-controlled study (APPLY-PNH) and a single-arm study (APPOINT-PNH).
APPLY-PNH: anti-C5 treatment experienced patients with PNH
APPLY-PNH enrolled adult PNH patients (RBC clone size ≥10%) with residual anaemia (haemoglobin <10 g/dl) despite previous treatment with a stable regimen of anti-C5 treatment (either eculizumab or ravulizumab) for at least 6 months prior to randomisation.
Patients (N=97) were randomised in 8:5 ratio either to receive iptacopan 200 mg orally twice daily (N=62) or to continue anti-C5 treatment (eculizumab N=23; or ravulizumab N=12) throughout the duration of the 24-week randomised controlled period (RCP). Randomisation was stratified based on prior anti-C5 treatment and transfusion history within the last 6 months.
Demographics and baseline disease characteristics were generally well balanced between treatment groups. At baseline, patients had a mean (standard deviation [SD]) age of 51.7 (16.9) years (range 22-84) and 49.8 (16.7) years (range 20-82) in the iptacopan and anti-C5 groups, respectively and 69% of patients were female in both groups. The mean (SD) haemoglobin was 8.9 (0.7) g/dl and 8.9 (0.9) g/dl, in the iptacopan and anti-C5 group, respectively. Fifty-seven percent (iptacopan group) and 60% (antiC5 group) of patients received at least one transfusion in the 6 months prior to randomisation. Amongst those, the mean (SD) number of transfusions was 3.1 (2.6) and 4.0 (4.3) in the iptacopan and anti-C5 group, respectively. The mean (SD) LDH level was 269.1 (70.1) U/l in the iptacopan group and 272.7 (84.8) U/l in the anti-C5 group. The mean (SD) absolute reticulocyte count was 193.2 (83.6) 109/l in the iptacopan group and 190.6 (80.9) 109/l in the anti-C5 group. The mean (SD) total PNH RBC clone size (Type II + III) was 64.6% (27.5%) in the iptacopan group and 57.4% (29.7%) in the anti-C5 group.
During the RCP, 1 patient in the iptacopan group discontinued treatment due to pregnancy; no patients in the anti-C5 group discontinued.
Efficacy was based on two primary endpoints to demonstrate superiority of iptacopan to anti-C5 in achieving haematological response after 24 weeks of treatment, without a need for transfusion, by assessing the proportion of patients demonstrating: 1) sustained increase of ≥2 g/dl in haemoglobin levels from baseline (haemoglobin improvement) and/or 2) sustained haemoglobin levels ≥12 g/dl.
Iptacopan demonstrated superiority to anti-C5 therapy for the two primary endpoints, as well as for several secondary endpoints including transfusion avoidance, changes from baseline in haemoglobin levels, Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores, absolute reticulocyte counts (ARCs) and annualised rate of clinical breakthrough haemolysis (see Table 2).
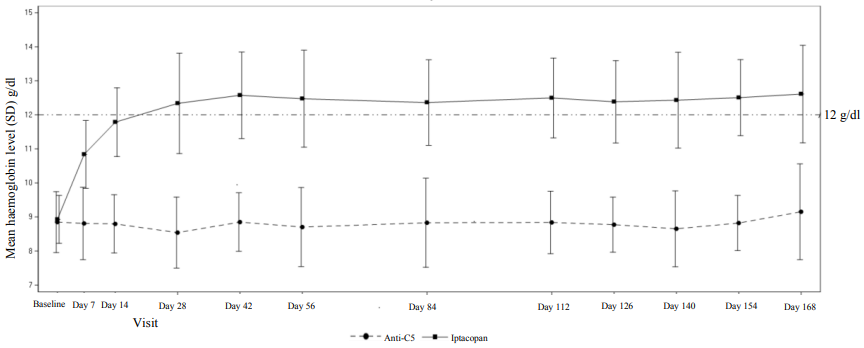
The treatment effect of iptacopan on haemoglobin was seen as early as day 7 and sustained during the study (see Figure 1).
Table 2. Efficacy results for the 24-week randomised treatment period in APPLY-PNH:
| Endpoints | Iptacopan (N=62) | Anti-C5 (N=35) | Difference (95% CI) p-value |
|---|---|---|---|
| Primary endpoints | |||
| Number of patients achieving haemoglobin improvement (sustained increase of haemoglobin levels ≥2 g/dl from baselinea in the absence of transfusions) | 51/60b | 0/35b | |
| Response ratec (%) | 82.3 | 2.0 | 80.2 (71.2, 87.6) <0.0001 |
| Number of patients achieving sustained haemoglobin level ≥12 g/dla in the absence of transfusions | 42/60b | 0/35b | |
| Response ratec (%) | 68.8 | 1.8 | 67.0 (56.4, 76.9) <0.0001 |
| Secondary endpoints | |||
| Number of patients avoiding transfusiond,e | 59/62b | 14/35b | |
| Transfusion avoidance ratec (%) | 94.8 | 25.9 | 68.9 (51.4, 83.9) <0.0001 |
| Haemoglobin level change from baseline (g/dl) (adjusted meanf) | 3.60 | -0.06 | 3.66 (3.20, 4.12) <0.0001 |
| FACIT-Fatigue score change from baseline (adjusted meang) | 8.59 | 0.31 | 8.29 (5.28, 11.29) <0.0001 |
| Clinical breakthrough haemolysish,i, % (n/N) | 3.2 (2/62) | 17.1 (6/35) | |
| Annualised rate of clinical breakthrough haemolysis | 0.07 | 0.67 | RR=0.10 (0.02, 0.61) 0.01 |
| Absolute reticulocyte count change from baseline (109/l) (adjusted meang) | -115.8 | 0.3 | -116.2 (-132.0, -100.3) <0.0001 |
| LDH ratio to baseline (adjusted geometric meang) | 0.96 | 0.98 | Ratio = 0.99 (0.89, 1.10) 0.84 |
| MAVEsh % (n/N) | 1.6 (1/62) | 0 | |
| Annualised rate of MAVEsh | 0.03 | 0 | 0.03 (-0.03, 0.10) 0.32 |
RR: rate ratio; LDH: lactate dehydrogenase; MAVEs: major adverse vascular events
a,d,h Assessed between days 126 and 168a, 14 and 168d, 1 and 168h.
b Based on observed data among evaluable patients. (In 2 patients with partially missing central haemoglobin data between days 126 and 168, the haematological response could not be established unequivocally. The haematological response was derived using multiple imputation. These patients did not discontinue.)
c Response rate reflects the model estimated proportion.
e Transfusion avoidance is defined as absence of administration of packed red blood cell transfusions between days 14 and 168 or meeting the criteria for transfusion between days 14 and 168.
f,g Adjusted mean assessed between days 126 and 168, values within 30 days after transfusion were excludedf/includedg in the analysis.
i Clinical breakthrough haemolysis is defined as meeting clinical criteria (either decrease of haemoglobin level ≥2 g/dl compared to the last assessment or within 15 days, or signs or symptoms of gross haemoglobinuria, painful crisis, dysphagia or any other significant clinical PNH-related signs and symptoms) and laboratory criteria (LDH >1.5 x ULN and increased as compared to the last 2 assessments).
Figure 1. Mean haemoglobin level* (g/dl) during 24-week randomised treatment period in APPLY-PNH:
* Note: The figure includes all haemoglobin data collected in the study, including those values within 30 days after RBC transfusion.
APPOINT-PNH: Complement inhibitor-naïve study
APPOINT-PNH was a single-arm study in 40 adult PNH patients (RBC clone size ≥10%) with haemoglobin <10 g/dl and LDH >1.5 x ULN who were not previously treated with a complement inhibitor. All 40 patients received iptacopan 200 mg orally twice daily during the 24-week open-label core treatment period.
At baseline, patients had a mean (SD) age of 42.1 (15.9) years (range 18-81) and 43% were female. The mean (SD) haemoglobin was 8.2 (1.1) g/dl. Seventy percent of patients received at least one transfusion in the 6 months prior to treatment. Amongst those the mean (SD) number of transfusions was 3.1 (2.1). The mean (SD) LDH level was 1 698.8 (683.3) U/l, and the mean (SD) absolute reticulocyte count was 154.3 (63.7) 109/l. The mean (SD) total PNH RBC clone size (Type II + III) was 42.7% (21.2%). No patients discontinued from the core treatment period of the study.
Efficacy was based on the primary endpoint assessing the effect of iptacopan treatment on the proportion of patients achieving haemoglobin improvement (sustained increase of ≥2 g/dl in haemoglobin levels from baseline, without a need for RBC transfusion, after 24 weeks).
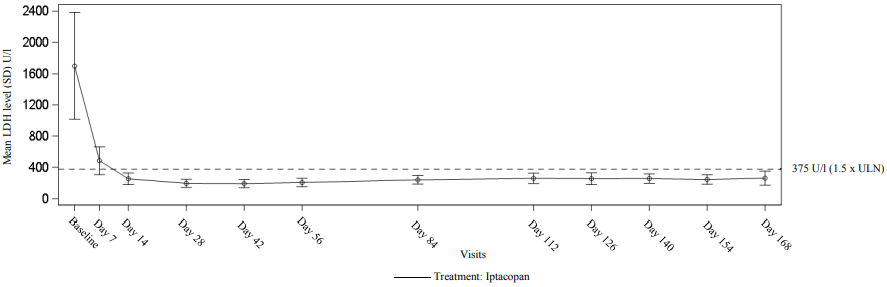
See Table 3 for detailed efficacy results and see Figure 2 for the mean LDH level change during the 24-week core treatment period.
Table 3. Efficacy results for the 24-week core treatment period in APPOINT-PNH:
| Endpoints | Iptacopan (N=40) 95% CI |
|---|---|
| Primary endpoint | |
| Number of patients achieving haemoglobin improvement (sustained increase of haemoglobin levels ≥2 g/dl from baselinea in the absence of transfusions) | 31/33b |
| Response ratec (%) | 92.2 (82.5, 100.0)d |
| Secondary endpoints | |
| Number of patients achieving sustained haemoglobin level ≥12 g/dla in the absence of transfusions | 19/33b |
| Response ratec (%) | 62.8 (47.5, 77.5) |
| Number of patients avoiding transfusione,f | 40/40b |
| Transfusion avoidance ratec (%) | 97.6 (92.5, 100.0) |
| Haemoglobin level change from baseline (g/dl) (adjusted meang) | +4.3 (3.9, 4.7) |
| Clinical breakthrough haemolysisi,j, % (n/N) | 0/40 |
| Annualised rate of clinical breakthrough haemolysis | 0.0 (0.0, 0.2) |
| Absolute reticulocyte count change from baseline (109/l) (adjusted meanh) | -82.5 (-89.3, -75.6) |
| LDH percent change from baseline (adjusted meanh) | -83.6 (-84.9, -82.1) |
| Percentage of patients with MAVEsj | 0.0 |
a,e,j Assessed between days 126 and 168a, 14 and 168e, 1 and 168j.
b Based on observed data among evaluable patients. (In 7 patients with partially missing central haemoglobin data between days 126 and 168, the haematological response could not be established unequivocally. The haematological response was derived using multiple imputation. These patients did not discontinue.)
c Response rate reflects the model estimated proportion.
d The threshold for demonstration of benefit was 15%, representing the rate that would have been expected on anti-C5 treatment.
f Transfusion avoidance is defined as absence of administration of packed red blood cell transfusions between days 14 and 168 or meeting the criteria for transfusion between days 14 and 168.
g,h Adjusted mean assessed between days 126 and 168, values within 30 days after transfusion were excludedg/includedh in the analysis.
i Clinical breakthrough haemolysis defined as meeting clinical criteria (either decrease of haemoglobin level ≥2 g/dl compared to the latest assessment or within 15 days; or signs or symptoms of gross haemoglobinuria, painful crisis, dysphagia or any other significant clinical PNH-related signs and symptoms) and laboratory criteria (LDH >1.5 x ULN and increased as compared to the last 2 assessments).
Figure 2. Mean LDH level (U/l) during 24-week core treatment period in APPOINT-PNH:
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with FABHALTA in one or more subsets of the paediatric population in PNH (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
Following oral administration, iptacopan reached peak plasma concentrations approximately 2 hours post dose. At the recommended dosing regimen of 200 mg twice daily, steady state is achieved in approximately 5 days with minor accumulation (1.4-fold). In healthy volunteers, steady-state Cmax,ss (geo-mean (CV)) was 4 020 ng/ml (23.8) and AUCtau,ss was 25 400 ng*hr/ml (15.2%). Inter- and intra-subject variability in iptacopan pharmacokinetics is low to moderate.
Results from a food-effect study with a high-fat high-calorie meal in healthy volunteers indicated that Cmax and area under the curve (AUC) of iptacopan were not affected by food. Therefore, iptacopan may be taken with or without food.
Distribution
Iptacopan showed concentration-dependent plasma protein binding due to binding to the target FB in the systemic circulation. Iptacopan was 75 to 93% protein bound in vitro at the relevant clinical plasma concentrations. After administration of iptacopan 200 mg twice daily, the geo-mean apparent volume of distribution at steady state was approximately 265 litres.
Biotransformation
Metabolism is a predominant elimination pathway for iptacopan, with approximately 50% of the dose attributed to oxidative pathways. Metabolism of iptacopan includes N-dealkylation, O-deethylation, oxidation and dehydrogenation, mostly driven by CYP2C8 with a small contribution from CYP2D6. Direct glucuronidation (by UGT1A1, UGT1A3 and UGT1A8) is a minor pathway. In plasma, iptacopan was the major component, accounting for 83% of the AUC0-48h. Two acyl glucuronides were the only metabolites detected in plasma and were minor, accounting for 8% and 5% of the AUC0-48h. Iptacopan metabolites are not considered pharmacologically active.
Elimination
In a study in healthy volunteers, following a single 100 mg oral dose of [14C]-iptacopan, mean total excretion of radioactivity (iptacopan and metabolites) was 71.5% in the faeces and 24.8% in the urine. Specifically, 17.9% of the dose was excreted as parent iptacopan in the urine and 16.8% in faeces. The apparent clearance (CL/F) after administration of iptacopan 200 mg twice daily at steady state is 7 960 ml/min. The half-life (t½) of iptacopan at steady state is approximately 25 hours after administration of iptacopan 200 mg twice daily.
Linearity/non-linearity
At doses between 25 and 100 mg twice daily, the pharmacokinetics of iptacopan were overall less than dose proportional. However, oral doses of 100 mg and 200 mg were approximately dose proportional. Non-linearity was primarily attributed to the saturable binding of iptacopan to its target FB in plasma.
Drug interactions
A dedicated interaction study in which iptacopan was co‑administered with other medicinal products was conducted in healthy volunteers and did not demonstrate any clinically relevant interactions.
Iptacopan as a substrate
CYP2C8 inhibitors:
When iptacopan is co‑administered with clopidogrel (a moderate CYP2C8 inhibitor), the iptacopan Cmax and the AUC increased by 5% and 36%, respectively.
OATP1B1/OATP1B3 inhibitors:
When iptacopan is co‑administered with ciclosporin (a strong OATP 1B1/1B3 inhibitor, and a PgP and BCRP inhibitor), the iptacopan Cmax and AUC increased by 41% and 50%, respectively.
Iptacopan as an inhibitor
PgP substrates:
In the presence of iptacopan, the Cmax of digoxin (a PgP substrate) increased by 8% while its AUC was unchanged.
OATP substrates:
In the presence of iptacopan, the Cmax and AUC of rosuvastatin (an OATP substrate) remained unchanged.
Special populations
A population pharmacokinetic (PK) analysis was conducted on data from 234 patients. Age (18 to 84 years), body weight, eGFR, race and gender did not significantly influence iptacopan PK. Studies that included Asian subjects showed that the PK of iptacopan were similar to Caucasian (white) subjects.
Renal impairment
The effect of renal impairment on the clearance of iptacopan was assessed using a population PK analysis. There were no clinically relevant differences in the clearance of iptacopan between patients with normal renal function and patients with mild (eGFR between 60 and 90 ml/min) or moderate (eGFR between 30 and 60 ml/min) renal impairment and no dose adjustment is required (see section 4.2). Patients with severe renal impairment or on dialysis have not been studied.
Hepatic impairment
Based on a study in subjects with mild (Child-Pugh A, n=8), moderate (Child-Pugh B, n=8) or severe (Child-Pugh C, n=6) hepatic impairment, a negligible effect on the total systemic exposure of iptacopan was observed compared to subjects with normal hepatic function. Unbound iptacopan Cmax increased 1.4-, 1.7- and 2.1-fold, and unbound iptacopan AUCinf increased by 1.5-, 1.6- and 3.7-fold in subjects with mild, moderate and severe hepatic impairment, respectively (see section 4.2).
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development.
Reproductive toxicity
In oral dose animal fertility studies, iptacopan did not impact fertility in male rats up to the highest dose tested (750 mg/kg/day), which corresponds to 6-fold the MRHD based on AUC. Reversible effects on the male reproductive system (testicular tubular degeneration and hypospermatogenesis) were observed in repeated dose toxicity studies after oral administration in rats and dogs at doses >3- fold the MRHD based on AUC, with no apparent effects on sperm numbers, morphology or motility, or fertility.
In the female fertility and early embryonic developmental study in rats, iptacopan-related findings were limited to increased pre-and post-implantation losses and, consequently, decreased numbers of live embryos only at the highest dose of 1 000 mg/kg/day orally, which corresponds to ~5-fold the MRHD based on total AUC. The dose of 300 mg/kg/day is the no-observed-adverse-effect level (NOAEL) which corresponds to ~2-fold the MRHD based on AUC.
Animal reproduction studies in rats and rabbits demonstrated that oral administration of iptacopan during organogenesis did not induce adverse embryo or foetal toxicity up to the highest doses, which correspond to 5-fold (for rats) and 8-fold (for rabbits) the MRHD of 200 mg twice daily based on AUC.
In the pre- and postnatal development study in rats, with iptacopan administered orally to females during gestation, parturition and lactation (from gestational day 6 to lactation day 21), there were no adverse effects on pregnant dams or offspring up to the highest dose tested of 1 000 mg/kg/day (estimated 5-fold the MRHD based on AUC).
Repeated dose toxicity
In the chronic toxicity study, one male dog at the highest dose level (margin to clinical exposure near 20-fold), was sacrificed 103 days after completed iptacopan administration due to irreversible nonregenerative severe anaemia associated with bone marrow fibrosis. During the treatment phase, haematology findings indicating inflammation and dyserythropoiesis were observed. No mechanism for the observed findings has been identified and a relation to treatment cannot be excluded.
Mutagenicity and carcinogenicity
Iptacopan was not genotoxic or mutagenic in a battery of in vitro and in vivo assays.
Carcinogenicity studies conducted with iptacopan in mice and rats via oral administration did not identify any carcinogenic potential. The highest doses of iptacopan studied in mice (1 000 mg/kg/day) and rats (750 mg/kg/day) were approximately 4- and 12-fold the MRHD based on AUC, respectively.
Phototoxicity
In vitro and in vivo phototoxicity tests were equivocal. In the in vivo phototoxicity study, with iptacopan at doses between 100 and 1 000 mg/kg (equivalent to 38-fold the human total Cmax at the MRHD), some mice showed a non-dose-response pattern of transient minimal erythema, scabs and dryness and slight increase in average ear weight subsequent to irradiation.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.