FASLODEX Solution for injection Ref.[8323] Active ingredients: Fulvestrant
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: AstraZeneca AB, SE-151 85 Södertälje, Sweden
Pharmacodynamic properties
Pharmacotherapeutic group: Endocrine therapy, Antiestrogens
ATC code: L02BA03
Mechanism of action and pharmacodynamic effects
Fulvestrant is a competitive estrogen receptor (ER) antagonist with an affinity comparable to estradiol. Fulvestrant blocks the trophic actions of estrogens without any partial agonist (estrogen-like) activity. The mechanism of action is associated with downregulation of estrogen receptor protein levels.
Clinical studies in postmenopausal women with primary breast cancer have shown that fulvestrant significantly downregulates ER protein in ER positive tumours compared with placebo. There was also a significant decrease in progesterone receptor expression consistent with a lack of intrinsic estrogen agonist effects. It has also been shown that fulvestrant 500 mg downregulates ER and the proliferation marker Ki67, to a greater degree than fulvestrant 250 mg in breast tumours in postmenopausal neoadjuvant setting.
Clinical efficacy and safety in advanced breast cancer
Monotherapy
A Phase 3 clinical study was completed in 736 postmenopausal women with advanced breast cancer who had disease recurrence on or after adjuvant endocrine therapy or progression following endocrine therapy for advanced disease. The study included 423 patients whose disease had recurred or progressed during antiestrogen therapy (AE subgroup) and 313 patients whose disease had recurred or progressed during aromatase inhibitor therapy (AI subgroup). This study compared the efficacy and safety of Faslodex 500 mg (n=362) with Faslodex 250 mg (n=374). Progression-free survival (PFS) was the primary endpoint; key secondary efficacy endpoints included objective response rate (ORR), clinical benefit rate (CBR) and overall survival (OS). Efficacy results for the CONFIRM study are summarized in Table 3.
Table 3. Summary of results of the primary efficacy endpoint (PFS) and key secondary efficacy endpoints in the CONFIRM study:
A Phase 3, randomised, double-blind, double-dummy, multicentre study of Faslodex 500 mg versus anastrozole 1 mg was conducted in postmenopausal women with ER-positive and/or PgR-positive locally advanced or metastatic breast cancer who had not previously been treated with any hormonal therapy. A total of 462 patients were randomised 1:1 sequentially to receive either fulvestrant 500 mg or anastrozole 1 mg. Randomisation was stratified by disease setting (locally advanced or metastatic), prior chemotherapy for advanced disease, and measurable disease.
The primary efficacy endpoint of the study was investigator assessed progression-free survival (PFS) evaluated according to RECIST 1.1 (Response Evaluation Criteria in Solid Tumours). Key secondary efficacy endpoints included overall survival (OS) and objective response rate (ORR).
Patients enrolled in this study had a median age of 63 years (range 36-90). The majority of patients (87.0%) had metastatic disease at baseline. Fifty-five percent (55.0%) of patients had visceral metastasis at baseline. A total of 17.1% of patients received a prior chemotherapy regimen for advanced disease; 84.2% of patients had measurable disease.
Consistent results were observed across the majority of pre-specified patient subgroups. For the subgroup of patients with disease limited to non-visceral metastasis (n=208), the HR was 0.592 (95% CI: 0.419, 0.837) for the Faslodex arm compared to the anastrozole arm. For the subgroup of patients with visceral metastasis (n=254), the HR was 0.993 (95% CI: 0.740, 1.331) for the Faslodex arm compared to the anastrozole arm. The efficacy results of the FALCON study are presented in Table 4 and Figure 1.
Table 4. Summary of results of the primary efficacy endpoint (PFS) and key secondary efficacy endpoints (Investigator Assessment, Intent-To-Treat Population) ─ FALCON study:
| Faslodex 500 mg (N=230) | Anastrozole 1 mg (N=232) | |
|---|---|---|
| Progression-Free Survival | ||
| Number of PFS Events (%) | 143 (62.2%) | 166 (71.6%) |
| PFS Hazard Ratio (95% CI) and p-value | HR 0.797 (0.637-0.999) p=0.0486 | |
| PFS Median [months (95% CI)] | 16.6 (13.8, 21.0) | 13.8 (12.0, 16.6) |
| Number of OS Events* | 67 (29.1%) | 75 (32.3%) |
| OS Hazard Ratio (95% CI) and p-value | HR 0.875 (0.629–1.217) p=0.4277 | |
| ORR** | 89 (46.1%) | 88 (44.9%) |
| ORR Odds Ratio (95% CI) and p-value | OR 1.074 (0.716–1.614) p=0.7290 | |
| Median DoR (months) | 20.0 | 13.2 |
| CBR | 180 (78.3%) | 172 (74.1%) |
| CBR Odds Ratio (95% CI) and p-value | OR 1.253 (0.815–1.932) p=0.3045 | |
* (31% maturity)-not final OS analysis
** for patients with measurable disease
Figure 1. Kaplan-Meier Plot of Progression-Free Survival (Investigator Assessment, Intent-To-Treat Population) ─ FALCON Study:
Two Phase 3 clinical studies were completed in a total of 851 postmenopausal women with advanced breast cancer who had disease recurrence on or after adjuvant endocrine therapy or progression following endocrine therapy for advanced disease. Seventy seven percent (77%) of the study population had estrogen receptor positive breast cancer. These studies compared the safety and efficacy of monthly administration of Faslodex 250 mg versus the daily administration of 1 mg anastrozole (aromatase inhibitor). Overall, Faslodex at the 250 mg monthly dose was at least as effective as anastrozole in terms of progression-free survival, objective response, and time to death. There were no statistically significant differences in any of these endpoints between the two treatment groups. Progression-free survival was the primary endpoint. Combined analysis of both studies showed that 83% of patients who received Faslodex progressed, compared with 85% of patients who received anastrozole. Combined analysis of both studies showed the hazard ratio of Faslodex 250 mg to anastrozole for progression-free survival was 0.95 (95% CI 0.82 to 1.10). The objective response rate for Faslodex 250 mg was 19.2% compared with 16.5% for anastrozole. The median time to death was 27.4 months for patients treated with Faslodex and 27.6 months for patients treated with anastrozole. The hazard ratio of Faslodex 250 mg to anastrozole for time to death was 1.01 (95% CI 0.86 to 1.19).
Combination therapy with palbociclib
A Phase 3, international, randomised, double-blind, parallel-group, multicentre study of Faslodex 500 mg plus palbociclib 125 mg versus Faslodex 500 mg plus placebo was conducted in women with HR-positive, HER2-negative locally advanced breast cancer not amenable to resection or radiation therapy with curative intent or metastatic breast cancer, regardless of their menopausal status, whose disease progressed after prior endocrine therapy in the (neo) adjuvant or metastatic setting.
A total of 521 pre/peri- and postmenopausal women who had progressed on or within 12 months from completion of adjuvant endocrine therapy on or within 1 month from prior endocrine therapy for advanced disease, were randomised 2:1 to Faslodex plus palbociclib or Faslodex plus placebo and stratified by documented sensitivity to prior hormonal therapy, menopausal status at study entry (pre/peri- versus postmenopausal), and presence of visceral metastases. Pre/perimenopausal women received the LHRH agonist goserelin. Patients with advanced/metastatic, symptomatic, visceral spread, that were at risk of life-threatening complications in the short term (including patients with massive uncontrolled effusions [pleural, pericardial, peritoneal], pulmonary lymphangitis, and over 50% liver involvement), were not eligible for enrolment into the study.
Patients continued to receive assigned treatment until objective disease progression, symptomatic deterioration, unacceptable toxicity, death, or withdrawal of consent, whichever occurred first. Crossover between treatment arms was not allowed.
Patients were well matched for baseline demographics and prognostic characteristics between the Faslodex plus palbociclib arm and the Faslodex plus placebo arm. The median age of patients enrolled in this study was 57 years (range 29, 88). In each treatment arm the majority of patients were White, had documented sensitivity to prior hormonal therapy, and were postmenopausal. Approximately 20% of patients were pre/perimenopausal. All patients had received prior systemic therapy and most patients in each treatment arm had received a previous chemotherapy regimen for their primary diagnosis. More than half (62%) had an ECOG PS of 0, 60% had visceral metastases, and 60% had received more than 1 prior hormonal regimen for their primary diagnosis.
The primary endpoint of the study was investigator-assessed PFS evaluated according to RECIST 1.1. Supportive PFS analyses were based on an Independent Central Radiology Review. Secondary endpoints included OR, CBR, OS, safety, and time-to-deterioration (TTD) in pain endpoint.
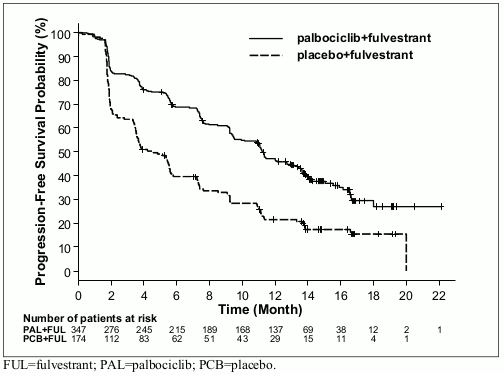
The study met its primary endpoint of prolonging investigator-assessed PFS at the interim analysis conducted on 82% of the planned PFS events; the results crossed the pre-specified Haybittle-Peto efficacy boundary (α=0.00135), demonstrating a statistically significant prolongation in PFS and a clinically meaningful treatment effect. A more mature update of efficacy data is reported in Table 5.
Table 5. Efficacy results – PALOMA3 study (Investigator assessment, intent-to-treat population):
| Updated Analysis (23 October 2015 cut-off) | ||
|---|---|---|
| Faslodex plus palbociclib (N=347) | Faslodex plus placebo (N=174) | |
| Progression-Free Survival | ||
| Median [months (95% CI)] | 11.2 (9.5, 12.9) | 4.6 (3.5, 5.6) |
| Hazard ratio (95% CI) and p-value | 0.497 (0.398, 0.620), p<0.000001 | |
| Secondary end points* | ||
| OR [% (95% CI)] | 26.2 (21.7, 31.2) | 13.8 (9.0, 19.8) |
| OR (measurable disease) [% (95% CI)] | 33.7 (28.1, 39.7) | 17.4 (11.5, 24.8) |
| CBR [% (95% CI)] | 68.0 (62.8, 72.9) | 39.7 (32.3, 47.3) |
* Response endpoints based on confirmed and unconfirmed responses.
N=number of patients; CI=confidence interval; OR=objective response; CBR=clinical benefit response
Figure 2. Kaplan-Meier plot of progression-free survival (investigator assessment, intent-to-treat population) – PALOMA3 study:
A reduction in the risk of disease progression or death in the Faslodex plus palbociclib arm was observed in all individual patient subgroups defined by stratification factors and baseline characteristics. This was evident for pre/perimenopausal women (HR of 0.46 [95% CI: 0.28, 0.75]) and postmenopausal women (HR of 0.52 [95% CI: 0.40, 0.66]) and patients with visceral site of metastatic disease (HR of 0.50 [95% CI: 0.38, 0.65]) and non-visceral site of metastatic disease (HR of 0.48 [95% CI: 0.33, 0.71]). Benefit was also observed regardless of lines of prior therapy in the metastatic setting, whether 0 (HR of 0.59 [95% CI: 0.37, 0.93]), 1 (HR of 0.46 [95% CI: 0.32, 0.64]), 2 (HR of 0.48 [95% CI: 0.30, 0.76]), or ≥3 lines (HR of 0.59 [95% CI: 0.28, 1.22]). Additional efficacy measures (OR and TTR) assessed in the sub-groups of patients with or without visceral disease are displayed in Table 6.
Table 6. Efficacy results in visceral and non-visceral disease from PALOMA3 study (intent-to-treat population):
|/2_. \2<>_.Visceral Disease|\2<>_.Non-visceral Disease
| Faslodex plus palbociclib (N=206) | Faslodex plus placebo (N=105) | Faslodex plus palbociclib (N=141) | Faslodex plus placebo (N=69) | |
|---|---|---|---|---|
| OR [% (95% CI)] | 35.0 (28.5, 41.9) | 13.3 (7.5, 21.4) | 13.5 (8.3, 20.2) | 14.5 (7.2, 25.0) |
| TTR*, Median [months (range)] | 3.8 (3.5, 16.7) | 5.4 (3.5, 16.7) | 3.7 (1.9, 13.7) | 3.6 (3.4, 3.7) |
* Response results based on confirmed and unconfirmed responses.
N=number of patients; CI=confidence interval; OR= objective response; TTR=time to first tumour response.
Patient-reported symptoms were assessed using the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ)-C30 and its Breast Cancer Module (EORTC QLQ-BR23). A total of 335 patients in the Faslodex plus palbociclib arm and 166 patients in the Faslodex plus placebo arm completed the questionnaire at baseline and at least 1 post-baseline visit.
Time-to-Deterioration was pre-specified as time between baseline and first occurrence of ≥10 points increase from baseline in pain symptom scores. Addition of palbociclib to Faslodex resulted in a symptom benefit by significantly delaying Time-to-Deterioration in pain symptom compared with Faslodex plus placebo (median 8.0 months versus 2.8 months; HR of 0.64 [95% CI: 0.49, 0.85]; p<0.001).
Effects on the postmenopausal endometrium
Preclinical data do not suggest a stimulatory effect of fulvestrant on the postmenopausal endometrium (see section 5.3). A 2-week study in healthy postmenopausal volunteers treated with 20 μg per day ethinylestradiol showed that pretreatment with Faslodex 250 mg resulted in significantly reduced stimulation of the postmenopausal endometrium, compared to pre-treatment with placebo, as judged by ultrasound measurement of endometrium thickness.
Neoadjuvant treatment for up to 16 weeks in breast cancer patients treated with either Faslodex 500 mg or Faslodex 250 mg did not result in clinically significant changes in endometrial thickness, indicating a lack of agonist effect. There is no evidence of adverse endometrial effects in the breast cancer patients studied. No data are available regarding endometrial morphology.
In two short-term studies (1 and 12 weeks) in premenopausal patients with benign gynaecologic disease, no significant differences in endometrial thickness were observed by ultrasound measurement between fulvestrant and placebo groups.
Effects on bone
There are no long-term data on the effect of fulvestrant on bone. Neoadjuvant treatment for up to 16 weeks in breast cancer patients with either Faslodex 500 mg or Faslodex 250 mg did not result in clinically significant changes in serum bone-turnover markers.
Paediatric population
Faslodex is not indicated for use in children. The European Medicines Agency has waived the obligation to submit the results of studies with Faslodex in all subsets of the paediatric population in breast cancer (see section 4.2 for information on paediatric use).
An open-label Phase 2 study investigated the safety, efficacy and pharmacokinetics of fulvestrant in 30 girls aged 1 to 8 years with Progressive Precocious Puberty associated with McCune Albright Syndrome (MAS). The paediatric patients received 4 mg/kg monthly intramuscular dose of fulvestrant. This 12-month study investigated a range of MAS endpoints and showed a reduction in the frequency of vaginal bleeding and a reduction in the rate of bone age advancement. The steady-state trough concentrations of fulvestrant in children in this study were consistent with that in adults (see section 5.2). There were no new safety concerns arising from this small study, but 5-year data are yet not available.
Pharmacokinetic properties
Absorption
After administration of Faslodex long-acting intramuscular injection, fulvestrant is slowly absorbed and maximum plasma concentrations (Cmax) are reached after about 5 days. Administration of Faslodex 500 mg regimen achieves exposure levels at, or close to, steady state within the first month of dosing (mean [CV]: AUC 475 [33.4%] ng.days/ml, Cmax 25.1 [35.3%] ng/ml, Cmin 16.3 [25.9%] ng/ml, respectively). At steady state, fulvestrant plasma concentrations are maintained within a relatively narrow range with up to an approximately 3-fold difference between maximum and trough concentrations. After intramuscular administration, the exposure is approximately dose-proportional in the dose range 50 to 500 mg.
Distribution
Fulvestrant is subject to extensive and rapid distribution. The large apparent volume of distributio Fulvestrant is highly (99%) bound to plasma proteins. Very low density lipoprotein (VLDL), low density lipoprotein (LDL), and high density lipoprotein (HDL) fractions are the major binding components. No interaction studies were conducted on competitive protein binding. The role of sex hormone-binding globulin (SHBG) has not been determined.
Biotransformation
The metabolism of fulvestrant has not been fully evaluated, but involves combinations of a number of possible biotransformation pathways analogous to those of endogenous steroids. Identified metabolites (includes 17-ketone, sulphone, 3-sulphate, 3- and 17-glucuronide metabolites) are either less active or exhibit similar activity to fulvestrant in antiestrogen models. Studies using human liver preparations and recombinant human enzymes indicate that CYP3A4 is the only P450 isoenzyme involved in the oxidation of fulvestrant; however, non-P450 routes appear to be more predominant in vivo. In vitro data suggest that fulvestrant does not inhibit CYP450 isoenzymes.
Elimination
Fulvestrant is eliminated mainly in metabolised form. The major route of excretion is via the faeces, with less than 1% being excreted in the urine. Fulvestrant has a high clearance, 11±1.7 ml/min/kg, suggesting a high hepatic extraction ratio. The terminal half-life (t1/2) after intramuscular administration is governed by the absorption rate and was estimated to be 50 days.
Special populations
In a population pharmacokinetic analysis of data from Phase 3 studies, no difference in fulvestrant’s pharmacokinetic profile was detected with regard to age (range 33 to 89 years), weight (40-127 kg) or race.
Renal impairment
Mild to moderate impairment of renal function did not influence the pharmacokinetics of fulvestrant to any clinically relevant extent.
Hepatic impairment
The pharmacokinetics of fulvestrant has been evaluated in a single-dose clinical study conducted in women with mild to moderate hepatic impairment (Child-Pugh class A and B). A high dose of a shorter duration intramuscular injection formulation was used. There was up to about 2.5-fold increase in AUC in women with hepatic impairment compared to healthy subjects. In patients administered Faslodex, an increase in exposure of this magnitude is expected to be well tolerated. Women with severe hepatic impairment (Child-Pugh class C) were not evaluated.
Paediatric population
The pharmacokinetics of fulvestrant has been evaluated in a clinical study conducted in 30 girls with Progressive Precocious Puberty associated with McCune Albright Syndrome (see section 5.1). The paediatric patients were aged 1 to 8 years and received 4 mg/kg monthly intramuscular dose of fulvestrant. The geometric mean (standard deviation) steady state trough concentration (Cmin,ss) and AUCss was 4.2 (0.9) ng/mL and 3680 (1020) ng*hr/mL, respectively. Although the data collected were limited, the steady-state trough concentrations of fulvestrant in children appear to be consistent with those in adults.
Preclinical safety data
The acute toxicity of fulvestrant is low.
Faslodex and other formulations of fulvestrant were well tolerated in animal species used in multiple dose studies. Local reactions, including myositis and granulomata at the injection site were attributed to the vehicle but the severity of myositis in rabbits increased with fulvestrant, compared to the saline control. In toxicity studies with multiple intramuscular doses of fulvestrant in rats and dogs, the antiestrogenic activity of fulvestrant was responsible for most of the effects seen, particularly in the female reproductive system, but also in other organs sensitive to hormones in both sexes. Arteritis involving a range of different tissues was seen in some dogs after chronic (12 months) dosing.
In dog studies following oral and intravenous administration, effects on the cardiovascular system (slight elevations of the S-T segment of the ECG [oral], and sinus arrest in one dog [intravenous]) were seen. These occurred at exposure levels higher than in patients (Cmax>15 times) and are likely to be of limited significance for human safety at the clinical dose.
Fulvestrant showed no genotoxic potential.
Fulvestrant showed effects upon reproduction and embryo/foetal development consistent with its antiestrogenic activity, at doses similar to the clinical dose. In rats, a reversible reduction in female fertility and embryonic survival, dystocia and an increased incidence of foetal abnormalities including tarsal flexure were observed. Rabbits given fulvestrant failed to maintain pregnancy. Increases in placental weight and post-implantation loss of foetuses were seen. There was an increased incidence of foetal variations in rabbits (backwards displacement of the pelvic girdle and 27 pre-sacral vertebrae).
A two-year oncogenicity study in rats (intramuscular administration of Faslodex) showed increased incidence of ovarian benign granulosa cell tumours in female rats at the high dose, 10 mg/rat/15 days and an increased incidence of testicular Leydig cell tumours in males. In a two-year mouse oncogenicity study (daily oral administration) there was an increased incidence of ovarian sex cord stromal tumours (both benign and malignant) at doses of 150 and 500 mg/kg/day. At the no-effect level for these findings, systemic exposure levels (AUC) were, in rats, approximately 1.5–fold the expected human exposure levels in females and 0.8-fold in males, and in mice, approximately 0.8-fold the expected human exposure levels in both males and females. Induction of such tumours is consistent with pharmacology-related endocrine feedback alterations in gonadotropin levels caused by antiestrogens in cycling animals. Therefore these findings are not considered to be relevant to the use of fulvestrant in postmenopausal women with advanced breast cancer.
Environmental Risk Assessment (ERA)
Environmental risk assessment studies have shown that fulvestrant may have potential to cause adverse effects to the aquatic environment (see section 6.6).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.