FINIDE Film-coated tablet Ref.[50319] Active ingredients: Finasteride
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2020 Publisher: Alphapharm Pty Ltd, Level 1, 30 The Bond, 30 – 34 Hickson Road, Millers Point NSW 2000, www.mylan.com.au
Product name and form
Finide – Finasteride.
| Pharmaceutical Form |
|---|
|
Finide 5 mg tablets are blue colored, oval shaped, biconvex, film-coated tablets, debossed with ‘FIN’ on one side and ‘5’ on the other side. |
Qualitative and quantitative composition
Finide film coated tablets contain 5 mg of finasteride as the active ingredient.
Excipients with known effect: Lactose monohydrate.
For the full list of excipients, see Section 6.1 LIST OF EXCIPIENTS.
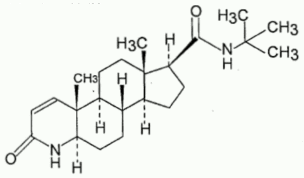
Chemical structure
Chemical name: N-(1,1-dimethylethyl)-3-oxo-4-aza-5-α-androst-1-ene-17-β-carboxamide
Molecular formula: C23H36N2O2
Molecular Weight: 372.55
CAS Number: 98319-26-7
| Active Ingredient | Description | |
|---|---|---|
| Finasteride |
Finasteride is a competitive inhibitor of human 5α-reductase, an intracellular enzyme which metabolises testosterone into the more potent androgen, dihydrotestosterone (DHT). In benign prostatic hyperplasia (BPH), enlargement of the prostate gland is dependent upon the conversion of testosterone to DHT within the prostate. Finasteride is highly effective in reducing circulating and intraprostatic DHT. Finasteride has no affinity for the androgen receptor. |
| List of Excipients |
|---|
|
Finide also contains the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, pregelatinised maize starch, sodium starch glycollate Type A, docusate sodium, magnesium stearate and Opadry complete film-coating system 03B50899 blue. |
Pack sizes and marketing
Available in blister packs containing 28 tablets (Al/Al) and 30 tablets (PVC/PE/PVDC/Al).
Some strengths, pack sizes and/or pack types may not be marketed.
Marketing authorization holder
Alphapharm Pty Ltd, Level 1, 30 The Bond, 30 – 34 Hickson Road, Millers Point NSW 2000, www.mylan.com.au
Marketing authorization dates and numbers
18/06/2010
Drugs
| Drug | Countries | |
|---|---|---|
| FINIDE | Australia, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.