FORTEO Solution for injection Ref.[10817] Active ingredients: Teriparatide
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
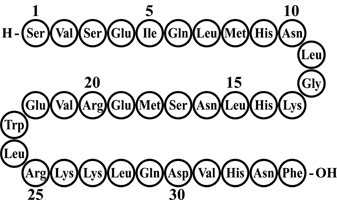
FORTEO (teriparatide injection) is a recombinant human parathyroid hormone analog (PTH 1-34). It has an identical sequence to the 34 N-terminal amino acids (the biologically active region) of the 84-amino acid human parathyroid hormone.
The molecular formula of teriparatide is C181H291N55O51S2 and molecular weight is 4117.8 daltons.
Its amino acid sequence is shown below:
Teriparatide is manufactured using a strain of Escherichia coli modified by recombinant DNA technology.
FORTEO is supplied as a sterile, colorless, clear, isotonic solution in a glass cartridge which is pre-assembled into a single-patient-use delivery device (pen) for subcutaneous injection. Each delivery device (pen) is filled with 2.7 mL to deliver 2.4 mL. Each mL contains 250 mcg of teriparatide (as a free base), 0.41 mg of glacial acetic acid, 0.1 mg of sodium acetate (anhydrous), 45.4 mg of mannitol, 3 mg of Metacresol, and Water for Injection. In addition, hydrochloric acid solution 10% and/or sodium hydroxide solution 10% may have been added to adjust the pH to 4.
Each prefilled delivery device (pen) delivers 20 mcg of teriparatide per dose for up to 28 days.
| Dosage Forms and Strengths |
|---|
|
Injection: 620 mcg/2.48 mL (250 mcg/mL) clear, colorless solution in a single-patient-use prefilled delivery device (pen) containing 28 daily doses of 20 mcg. |
| How Supplied |
|---|
|
FORTEO (teriparatide injection) is a clear and colorless solution, available as single-patient-use prefilled delivery device (pen) in the following package size:
Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA, www.forteo.com |
Drugs
| Drug | Countries | |
|---|---|---|
| FORTEO | Australia, Brazil, Canada, Hong Kong, Israel, Japan, New Zealand, Singapore, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.