FRENOLYN Inhalation powder Ref.[28222] Active ingredients: Budesonide
Source: Υπουργείο Υγείας (CY) Revision Year: 2017 Publisher: MEDOCHEMIE LTD, 1-10 Constantinoupoleos street, 3011 Limassol, Cyprus
4.1. Therapeutic indications
Indicated in patients with bronchial asthma.
4.2. Posology and method of administration
Posology
General Dosage Considerations
In the transfer of patients from the use of other devices, irrespective of whether twice daily or once daily dosing is to be used, the treatment should be individualised. Consideration should be given to the drug and to the method of delivery.
In all cases, the dosage should be reduced to the minimum necessary to maintain good asthma control.
Following each administration, patients should be instructed to clean their teeth and/or wash their mouth out with water to reduce the possibility of an oral Candida infection or hoarseness.
All patients, but especially those on once daily therapy, should be instructed that if their asthma worsens, for example persistent respiratory symptoms or greater use of bronchodilator, to double their budesonide dose, by administering the same dose twice daily, as appropriate for their dosing regimen, and to contact their physician as soon as possible.
To enhance therapeutic effect in patients when clinically required, an increased inhaled dose of budesonide is recommended, due to the reduced risk of systemic effects in comparison with combined therapy with oral glucocorticosteroids (see '‘Asthma’' section below).
The importance of regular administration of prophylactic therapy, even when asymptomatic, should be emphasised to all patients.
Patients should additionally have available a short acting inhaled bronchodilator for the relief of acute asthma.
Asthma
Frenolyn may permit replacement or significant reduction in dosage of oral glucocorticosteroids while maintaining asthma control. When transferral from oral steroids to budesonide is started, the patient should be in a relatively stable phase. A high dose of budesonide is then given in combination with the previously used oral steroid dose for about 10 days.
After that, the oral steroid dose should be gradually reduced (by for example 2.5 milligrams prednisolone or the equivalent each month) to the lowest possible level. In many cases, it is possible to completely substitute the oral steroid with inhaled budesonide. For further information on the withdrawal of corticosteroids, see section 4.4.
Twice Daily Administration
Adults
The dose should be administered in two divided doses, morning and evening, at approximately the same times each day.
For initiating treatment, during episodes of severe asthma and while discontinuing or reducing oral glucocorticosteroids, the adult dose should be 200 – 1600 micrograms daily in divided doses.
In less severe adult cases, and in children aged over twelve years, the recommended dose is 200 – 800 micrograms daily in divided doses. During periods of severe asthma the daily dose may be increased to a maximum of 1600 micrograms administered as divided doses.
Paediatric population
Children aged over twelve (12) years: The recommended adult dose may be used.
Children aged five (5) to twelve (12) years: The recommended dose is 200–800 micrograms daily in divided doses. During periods of severe asthma the daily dose may be increased to a maximum of 800 micrograms administered as divided doses.
Elderly
The recommended adult dose may be used; dosage adjustment is unnecessary.
Hepatic impairment
Although budesonide undergoes extensive hepatic first pass metabolism, no dosage adjustment is required.
Renal impairment
No dosage adjustment is required.
Once Daily Administration
General considerations
Once daily dosing transfer should be effectuated at the same equivalent total daily dose, and the drug and the method of delivery should be taken into consideration. Subsequently the dose should be titrated downward to the minimum dose required for the maintenance of effective asthma control.
The once daily dose should be administered in the evening, and the importance of taking the dose consistently and at the same time each evening should be emphasised to the patient.
Recommendations for the transfer of patients from the newer inhaled corticosteroids to once daily budesonide cannot be made, as there is insufficient data available.
Adults
For patients with mild to moderate asthma who have not previously received glucocorticosteroid therapy, by inhalation a dose of 200 – 400 micrograms per day is recommended.
For patients with mild to moderate asthma already controlled on inhaled steroids, such as budesonide or beclomethasone dipropionate, a dose up to 800 micrograms is recommended, administered twice daily.
Paediatric population
Children aged over twelve (12) years: The recommended adult dose may be used.
Children aged five (5) to twelve (12) years: Patients with mild to moderate asthma who have not previously received glucocorticosteroid by inhalation or who are already controlled on inhaled steroids, such as budesonide or beclomethasone dipropionate, a dose of 200-400 micrograms is recommended to be administered twice daily.
Elderly
The recommended adult dose may be used; dosage adjustment is unnecessary.
Hepatic impairment
Although budesonide undergoes extensive hepatic first pass metabolism, no dosage adjustment is required.
Renal impairment
No dosage adjustment is required.
Method of administration
Frenolyn is for oral inhalation only.
For instructions on the use of the medicinal product before administration, see section 6.6.
4.9. Overdose
Symptoms
Inhalation of large amounts of budesonide in a short period would result in suppression of the hypothalamic – pituitary – adrenal function.
Management
No specific treatment or emergency action is required. Treatment with inhaled budesonide at the required dose adequately to control asthma should be continued.
6.3. Shelf life
48 months.
6.4. Special precautions for storage
Store below 25°C in the original package, in order to protect from moisture.
Do not refrigerate or freeze.
Keep the container tightly closed.
In order to protect from moisture, always replace the inhaler in the outer case, and keep screw closed immediately after every use.
6.5. Nature and contents of container
Miat–Haler:
Polypropylene container consisting of a brown screw cap and a grey case. Removal of the cap reveals the body of the inhaler, consisting of a polyacetal (POM) mouthpiece, acrilonitrile butadiene styrene (ABS) body, a polybutylene terephthalate (PBT) dosing mechanism, and the immediate drug container made of methacrylate butadiene styrene (MABS) and sealed with a low density polyethylene (LDPE) stopper.
The grey case also contains a desiccant pill, with a symbol indicating not for oral ingestion. This should not be removed.
Each Miat–Haler is packed into a polyester – aluminium – polyethylene strip, and packaged, with a patient information leaflet, in a card carton.
Miat–Haler budesonide 200 is available in devices with 100 doses, or 200 doses.
Cartons containing one inhaler, or three inhalers are available.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
The Frenolyn Miat–Haler inhaler is packed in an aluminium blister in the outer carton. You should not open the blister or remove the inhaler from it until you need to use the inhaler. Open the aluminium blister and discard it. The Frenolyn Miat–Haler inhaler may rattle when you shake it, this does not mean anything is wrong; it is part of the mechanism. The inhaler is called a Miat–Haler. The Miat–Haler is easy to use, provided you follow the instructions. Your doctor or pharmacist should have shown you how to use the Miat–Haler. If you are uncertain how to use the Miat–Haler, ask your doctor or pharmacist.
The Miat-Haler consists of two pieces, as shown: It is a light grey, except for the cap, which is dark brown.
STEP 1:
Hold the Miat-Haler upright. Unscrew the inhaler by rotating anticlockwise, keeping the brown top in the upright position and remove it from the protective case.
Note: If the inhaler is not kept upright, a warning signal in the Inhaler will generate a rattling noise, reminding you to keep the Inhaler in the right position. If you hear the rattling noise repeat the operation keeping the Inhaler upright.
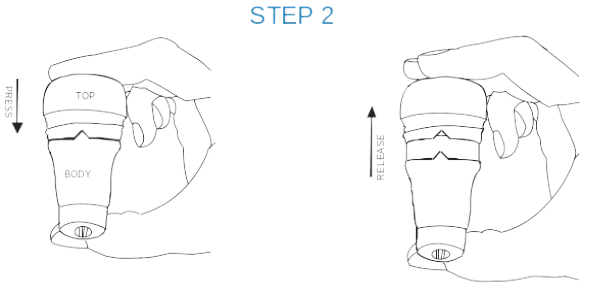
STEP 2:
Be sure that the Δ shaped notch on the body is aligned with the Λ notch οn the top.
If the notches are not aligned, turn the Inhaler until the notches are aligned.
Press the brown top and release. The dose is now ready for administration.
Note: At this stage the desired dosage of active is prepared for intake and ready for the next step (inhalation).
Attention: Proceed immediately to the next step. Do not interrupt the sequence at this stage.
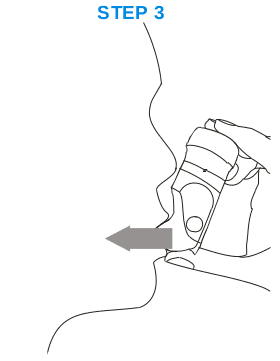
STEP 3:
Breathe out gently, before bringing the Inhaler to your mouth. Place the Inhaler mouthpiece between your lips (with the inhaler still kept in the upright position) and breathe in as deeply as possible. Remove the inhaler from your mouth and hold breath for a few seconds before breathing out.
You can repeat step 3 to be sure you have taken your entire dose.
Note: Α subtle sweet taste of the powder inhaled can be felt, that confirms you have inhaled the dose. You may not notice any taste in your mouth when using FRENOLYN Miat Haler, but you will still have received the correct dose.
Attention: After taking your dose, you should rinse your mouth out with water, and/or clean your teeth. This will reduce the risk of you getting “thrush” (a yeast infection) in your mouth.
You should wipe the mouthpiece with a clean dry tissue after each use.
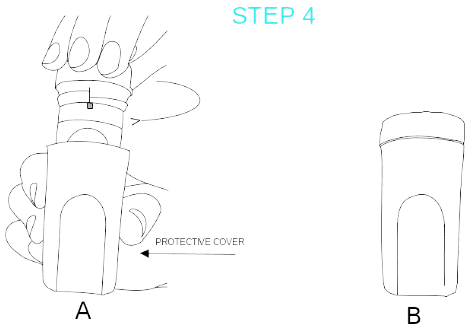
STEP 4:
Place the inhaler in the protective case and screw closed rotating clockwise.
Notice: If you need to take another inhalation, you should repeat steps 1-4 again.
Attention: Always place the inhaler back in its protective case after every use; this is necessary for the preparation of the next dose.
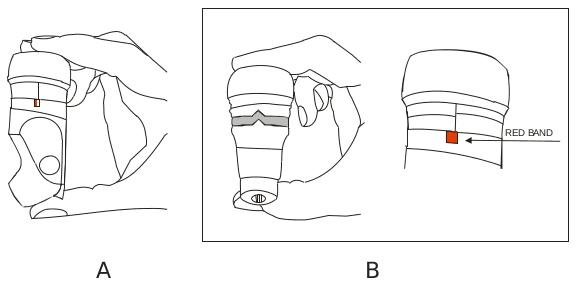
REPLACΊNG YOUR FRENOLYN:
As your inhaler becomes nearly empty, a red band will begin to appear in the small window on the right hand side of the device. When it is empty, after the labeled number of doses has been used, the red band will fill the window. The brown top will not depress, as it will lock to stop you using an empty inhaler.
You should see your doctor for a replacement prescription when you first see the red band appear.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.