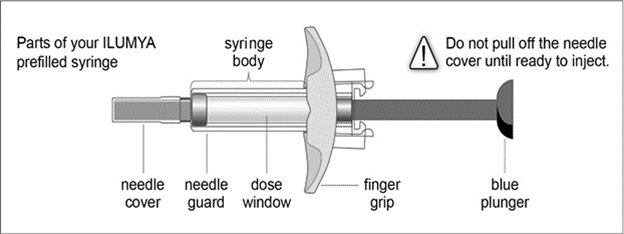
ILUMYA Solution for injection Ref.[27742] Active ingredients: Tildrakizumab
Source: FDA, National Drug Code (US) Revision Year: 2021
1. Indications and Usage
ILUMYA is indicated for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
2. Dosage and Administration
2.1 Dosage
ILUMYA is administered by subcutaneous injection. The recommended dose is 100 mg at Weeks 0, 4, and every twelve weeks thereafter. Each syringe contains 1 mL of 100 mg/mL tildrakizumab-asmn.
2.2 Tuberculosis Assessment Prior to Initiation of ILUMYA
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with ILUMYA [see Warnings and Precautions (5.3)].
2.3 Important Administration Instructions
ILUMYA should only be administered by a healthcare provider. Administer ILUMYA subcutaneously. Each pre-filled syringe is for single-dose only. Inject the full amount (1 mL), which provides 100 mg of tildrakizumab per syringe. If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regularly scheduled interval.
2.4 Preparation and Administration of ILUMYA
Before injection, remove ILUMYA carton from the refrigerator, and let the prefilled syringe (in the ILUMYA carton with the lid closed) sit at room temperature for 30 minutes.
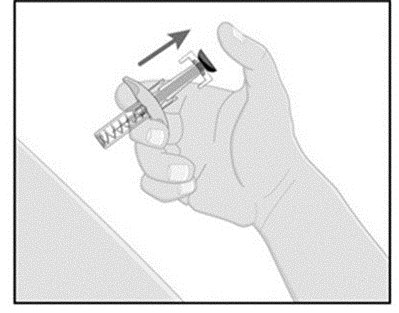
Follow the instructions on the ILUMYA carton to remove the prefilled syringe correctly, and remove only when ready to inject. Do not pull off the needle cover until you are ready to inject.
Inspect ILUMYA visually for particulate matter and discoloration prior to administration. ILUMYA is a clear to slightly opalescent, colorless to slightly yellow solution. Do not use if the liquid contains visible particles or the syringe is damaged. Air bubbles may be present; there is no need to remove them.
Choose an injection site with clear skin and easy access (such as abdomen, thighs, or upper arm). Do not administer 2 inches around the navel or where the skin is tender, bruised, erythematous, indurated, or affected by psoriasis. Also do not inject into scars, stretch marks, or blood vessels.
- While holding the body of the syringe, pull the needle cover straight off (do not twist) and discard.
- Inject ILUMYA subcutaneously as recommended [see Dosage and Administration (2.3)].
- Press down the blue plunger until it can go no further. This activates the safety mechanism that will ensure full retraction of the needle after the injection is given.
- Remove the needle from the skin entirely before letting go of the blue plunger. After the blue plunger is released, the safety lock will draw the needle inside the needle guard.
- Discard of any unused portion. Dispose of used syringe.
10. Overdosage
In the event of overdosage, monitor the patient for any signs or symptoms of adverse reactions and administer appropriate symptomatic treatment immediately.
16.2. Storage and Handling
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light until the time of use. Do not freeze. Do not shake. ILUMYA can be kept at room temperature at 25°C (77°F) for up to 30 days in the original carton to protect from light. Once stored at room temperature, do not place back in the refrigerator. If not used within 30 days, discard ILUMYA. Do not store ILUMYA above 25°C (77°F).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.