INCRELEX Solution for injection Ref.[9554] Active ingredients: Mecasermin
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Ipsen Pharma, 65, quai Georges Gorse, 92100, Boulogne-Billancourt, France
Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
INCRELEX is contraindicated in children and adolescents with active or suspected neoplasia, or any condition or medical history which increases the risk of benign or malignant neoplasia. Therapy should be discontinued if evidence of neoplasia develops.
As INCRELEX contains benzyl alcohol, it must not be given to premature babies or neonates.
Special warnings and precautions for use
Benign and malignant neoplasms
There is an increased risk of benign and malignant neoplasia in children and adolescents treated with INCRELEX, since IGF-1 plays a role in the initiation and progression of benign and malignant tumours.
There have been post-marketing reports of both benign and malignant neoplasms in children and adolescents who have received treatment with INCRELEX. These cases represented a variety of different malignancies and included rare malignancies usually not seen in children (see section 4.8). The increased risk of neoplasia may be higher in patients who receive INCRELEX for unapproved uses or at higher than recommended doses. Current knowledge of IGF-1 biology suggests that IGF-1 plays a role in malignancies in all organs and tissues. Physicians should therefore be vigilant of any symptoms of potential malignancy. If benign or malignant neoplasia develops, INCRELEX treatment should be discontinued definitely and appropriate expert medical care sought.
Mecasermin is not a substitute for GH treatment.
Mecasermin should not be used for growth promotion in patients with closed epiphyses.
Mecasermin should be administered shortly before or after a meal or snack, because it may have insulin-like hypoglycaemic effects. Special attention should be paid to young children, children with a history of hypoglycaemia and children with inconsistent food intake. Patients should avoid engaging in any high-risk activities within 2-3 hours after dosing, particularly at the initiation of mecasermin treatment, until a well- tolerated dose of INCRELEX has been established. If a person with severe hypoglycemia is unconscious or otherwise unable to ingest food normally, an injection of glucagon may be required. Persons with a history of severe hypoglycemia should have glucagon available. At the time of initial prescription, physicians should educate parents on the signs, symptoms and treatment of hypoglycaemia, including injection of glucagon.
Doses of insulin and/or other hypoglycaemic medicinal products may need to be reduced for diabetic subjects using this medicinal product.
Echocardiogram is recommended before initiation of mecasermin treatment in all patients. Patients who terminate treatment should also have an echocardiogram. Patients with abnormal echocardiogram findings or cardiovascular symptoms should be followed regularly with echocardiogram procedures.
Lymphoid tissue (e.g., tonsillar) hypertrophy associated with complications, such as snoring, sleep apnoea, and chronic middle-ear effusions have been reported with the use of this medicinal product. Patients should have examinations periodically and at the occurrence of clinical symptoms to rule out such potential complications or to initiate appropriate treatment.
Intracranial hypertension (IH) with papilloedema, visual changes, headache, nausea and/or vomiting has been reported in patients treated with mecasermin, as has been reported with therapeutic GH administration. IH-associated signs and symptoms resolved after interruption of dosing. Funduscopic examination is recommended at the initiation, periodically during the course of mecasermin therapy and at the occurrence of clinical symptoms.
Slipped capital femoral epiphysis (with the potential to lead to avascular necrosis) and progression of scoliosis can occur in patients who experience rapid growth. These conditions and other symptoms and signs known to be associated with GH treatment in general should be monitored during mecasermin treatment. Any patient with the onset of a limp or complaint of hip or knee pain should be evaluated.
In post-marketing experience in patients treated with INCRELEX, cases of hypersensitivity, urticaria, pruritus and erythema have been reported. These have been observed both as being systemic and/or local to the injection site. A small number of cases indicative of anaphylaxis requiring hospitalisation have been reported. Parents and patients should be informed that such reactions are possible and that if a systemic allergic reaction occurs, treatment should be interrupted and prompt medical attention should be sought.
Treatment should be reconsidered if after a year patients remain non-responsive.
Persons who have allergic reactions to injected IGF-1, who have unexpectedly high blood values of IGF-1 after injection, or who fail to show a growth response without any identified cause may be having an antibody response to injected IGF-1. This may be through the production of anti-IGF-1 IgEs, sustaining antibodies or neutralizing antibodies respectively. In such instances, instructions for antibody testing should be considered.
Excipients
INCRELEX contains 9 mg/ml benzyl alcohol as a preservative.
Benzyl alcohol may cause toxic reactions and anaphylactoid reactions in infants and children up to 3 years old.
This medicinal product contains less than 1 mmol sodium (23 mg) per vial, i.e. essentially ‘sodium-free’.
Traceability
In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded.
Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
Doses of insulin and/or other hypoglycaemic medicinal products may need to be reduced (see section 4.4).
Fertility, pregnancy and lactation
Women of childbearing potential / Contraception in males and females
A negative pregnancy test is recommended for all women of child bearing potential prior to treatment with mecasermin. It is also recommended that all women of childbearing potential use adequate contraception during treatment.
Pregnancy
There are no or limited amount of data for the use of mecasermin in pregnant women.
Animal studies are insufficient with respect to reproductive toxicity (see section 5.3). The potential risk for humans is unknown.
This medicinal product should not be used during pregnancy unless clearly necessary.
Breast-feeding
Breast-feeding while taking INCRELEX is not recommended, because there is insufficient information on the excretion of mecasermin in human milk.
Fertility
Mecasermin has been tested in a rat teratology study with no effects on fœtus up to 16 mg/kg (20 fold the maximum recommended human dose (MRHD) based on body surface area) and in a rabbit teratology with no effects on foetus at dose of 0.5 mg/kg (2 fold the MRHD based on body surface area). Mecasermin has no effects on fertility in rats using intravenous doses 0.25, 1, and 4 mg/day (up to 4 times the clinical exposure with the MRHD based on AUC).
The effects of mecasermin on the unborn child have not been studied. Therefore there is insufficient medical information to determine whether there are significant risks to a foetus. Studies have not been conducted with mecasermin in breast-feeding mothers. INCRELEX should not be given to pregnant or nursing women. A negative pregnancy test and adequate contraception is required in all pre-menopausal women receiving INCRELEX.
Effects on ability to drive and use machines
INCRELEX may have a major influence on the ability to drive or use machines in case of a hypoglycaemic episode. Hypoglycaemia is a very common adverse reaction.
Undesirable effects
Summary of the safety profile
Adverse reaction data was taken from a total of 413 clinical trial patients with severe Primary IGFD. Data was also collected from post-marketing sources.
The most frequently reported adverse reactions from the clinical trials were headache (44%), hypoglycaemia (28%), vomiting (26%), injection site hypertrophy (17%), and otitis media (17%).
Intracranial hypertension/increased intracranial pressure occurred in 4 (0.96%) of patients from the clinical trials and occurred in 7–9 year old treatment naïve subjects.
During clinical trials in other indications totaling approximately 300 patients, reports of local and/or systemic hypersensitivity were received for 8% of patients. There were also reports of systemic hypersensitivity from post-marketing use, of which some cases were indicative of anaphylaxis. Post-marketing reports of local allergic reactions were also received.
Some patients may develop antibodies to mecasermin. No attenuation of growth was observed as a consequence of the development of antibodies.
Tabulated list of adverse reactions
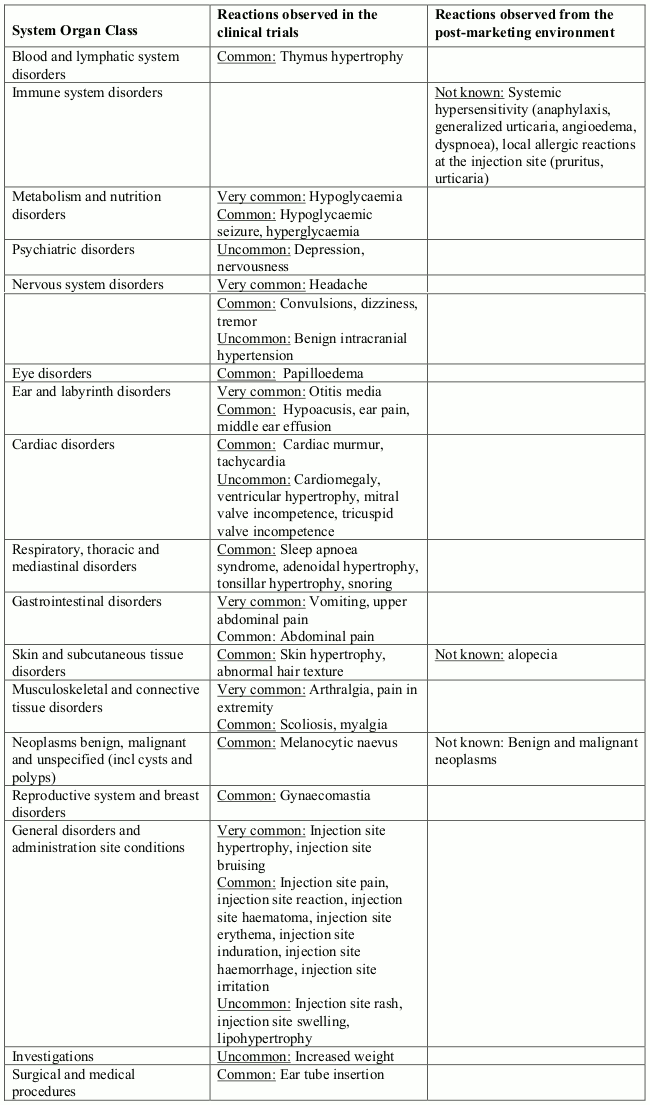
Table 1 contains very common (≥1/10), common (≥1/100 to <1/10) and uncommon (≥1/1000, <1/100) adverse reactions which occurred in clinical trials. Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. Other adverse reactions have been identified during post approval use of INCRELEX. As these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency (not known).
Table 1. Adverse reactions:
Description of selected adverse reactions
Neoplasms
There have been post-marketing reports of benign and malignant neoplasms in children and adolescents who have received treatment with INCRELEX. These cases represented a variety of different malignancies and included rare malignancies usually not seen in children (see section 4.4 and 4.3).
Systemic/local hypersensitivity
Clinical Trial
During clinical trials in other indications (totaling approximately 300 patients) 8% of patients reported a local and/or systemic hypersensitivity reactions. All cases were mild or moderate in severity and none was serious.
Post-marketing reports
Systemic hypersensitivity included symptoms such as anaphylaxis, generalized urticaria, angioedema and dyspnoea. The symptoms in the cases indicative of anaphylaxis included hives, angioedema and dyspnoea. Some patients required hospitalization. Upon re-administration, symptoms did not re-occur in all patients. There were also reports of local allergic reactions at the injection site. Typically these were pruritus and urticaria.
Hypoglycaemia
Of the 115 (28%) subjects who experienced one or more episode of hypoglycaemia, 6 subjects experienced a hypoglycaemic seizure on one or more occasion. Symptomatic hypoglycaemia was generally avoided when a meal or snack was consumed either shortly before or after the administration of INCRELEX.
Injection site hypertrophy
This reaction occurred in 71 (17%) subjects from the clinical trials and was generally associated with lack of proper rotation of injections. When injections were properly dispersed, the condition resolved.
Tonsillar hypertrophy
This was noted in 38 (9%) subjects, particularly in the first 1 to 2 years of therapy with lesser tonsillar growth in subsequent years.
Snoring
This occurred generally in the first year of treatment and was reported in 30 subjects (7%).
Intracranial hypertension/increased intracranial pressure
This occurred in 4 subjects (0.96%); in two subjects INCRELEX was discontinued and not restarted; in two subjects the event did not recur after restarting INCRELEX at a reduced dose. All 4 subjects recovered from the event without sequelae.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.