INTELENCE Tablet Ref.[8777] Active ingredients: Etravirine
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium
Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Co-administration with elbasvir/grazoprevir (see section 4.5).
Special warnings and precautions for use
INTELENCE should optimally be combined with other antiretrovirals that exhibit activity against the patient’s virus (see section 5.1).
A decreased virologic response to etravirine was observed in patients with viral strains harbouring 3 or more among the following mutations V90I, A98G, L100I, K101E/P, V106I, V179D/F, Y181C/I/V, and G190A/S (see section 5.1).
Conclusions regarding the relevance of particular mutations or mutational patterns are subject to change with additional data, and it is recommended to always consult current interpretation systems for analysing resistance test results.
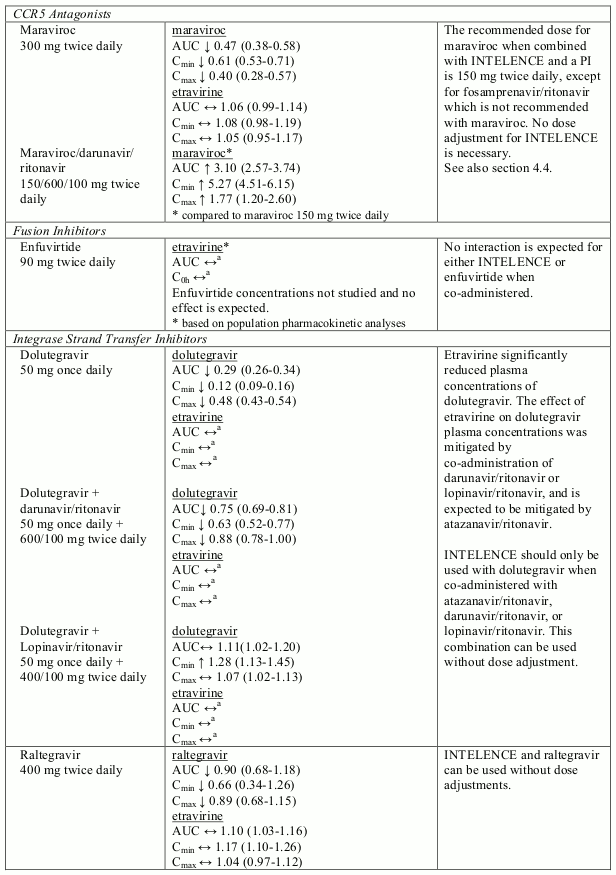
No data other than drug-drug interaction data (see section 4.5) are available when etravirine is combined with raltegravir or maraviroc.
Severe cutaneous and hypersensitivity reactions
Severe cutaneous adverse reactions have been reported with etravirine. In clinical trials, Stevens-Johnson Syndrome and erythema multiforme have been rarely (<0.1%) reported. Treatment with INTELENCE should be discontinued if a severe cutaneous reaction develops.
The clinical data are limited and an increased risk of cutaneous reactions in patients with a history of NNRTI-associated cutaneous reactions cannot be excluded. Caution should be observed in such patients, especially in case of history of a severe cutaneous drug reaction.
Cases of severe hypersensitivity syndromes, including DRESS (Drug Rash with Eosinophilia and Systemic Symptoms) and TEN (toxic epidermal necrolysis), sometimes fatal, have been reported with the use of etravirine (see section 4.8). The DRESS syndrome is characterised by rash, fever, eosinophilia and systemic involvement (including, but not limited to, severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis and eosinophilia). Time to onset is usually around 3-6 weeks and the outcome in most cases is favourable upon discontinuation and after initiation of corticosteroid therapy.
Patients should be informed to seek medical advice if severe rash or hypersensitivity reactions occur. Patients who are diagnosed with a hypersensitivity reaction whilst on therapy must discontinue INTELENCE immediately.
Delay in stopping INTELENCE treatment after the onset of severe rash may result in a life-threatening reaction.
Patients who have stopped treatment due to hypersensitivity reactions should not restart therapy with INTELENCE.
Rash
Rash has been reported with etravirine. Most frequently, rash was mild to moderate, occurred in the second week of therapy, and was infrequent after week 4. Rash was mostly self-limiting and generally resolved within 1 to 2 weeks on continued therapy. When prescribing INTELENCE to females, prescribers should be aware that the incidence of rash was higher in females (see section 4.8).
Paediatric population
For children who cannot swallow the tablet(s) whole, the tablet(s) may be dispersed in liquid. This should only be considered if the child is likely to take the entire dose of the tablet(s) in liquid (see sections 4.2 and 6.6). The importance of consuming the entire dose needs to be highlighted to the child and his/her caregiver to avoid too low exposure and lack of virologic response. In case of any doubt that a child will take the entire dose of the tablet(s) dispersed in liquid, treatment with another antiretroviral product needs to be considered.
Elderly
Experience in geriatric patients is limited: in the Phase III trials, 6 patients aged 65 years or older and 53 patients aged 56-64 years received etravirine. The type and incidence of adverse reactions in patients >55 years of age were similar to the ones in younger patients (see sections 4.2 and 5.2).
Pregnancy
Given the increased etravirine exposure during pregnancy, caution should be applied for those pregnant patients that require concomitant medicinal products or have comorbidities that may further increase etravirine exposure.
Patients with coexisting conditions
Hepatic impairment
Etravirine is primarily metabolised and eliminated by the liver and highly bound to plasma proteins. Effects on unbound exposure could be expected (has not been studied) and therefore caution is advised in patients with moderate hepatic impairment. Etravirine has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) and its use is therefore not recommended in this group of patients (see sections 4.2 and 5.2).
Co-infection with HBV (hepatitis B virus) or HCV (hepatitis C virus)
Caution should be exercised in patients co-infected with hepatitis B or C virus due to the current limited data available. A potential increased risk of liver enzymes increase cannot be excluded.
Weight and metabolic parameters
An increase in weight and in levels of blood lipids and glucose may occur during antiretroviral therapy. Such changes may in part be linked to disease control and life style. For lipids, there is in some cases evidence for a treatment effect, while for weight gain there is no strong evidence relating this to any particular treatment. For monitoring of blood lipids and glucose reference is made to established HIV treatment guidelines. Lipid disorders should be managed as clinically appropriate.
Immune reconstitution syndrome
In HIV infected patients with severe immune deficiency at the time of initiation of CART, an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first weeks or months of initiation of CART. Relevant examples are cytomegalovirus retinitis, generalised and/or focal mycobacterial infections and Pneumocystis jiroveci pneumonia. Any inflammatory symptoms should be evaluated and treatment instituted when necessary.
Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported to occur in the setting of immune reactivation; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment (see section 4.8).
Osteonecrosis
Although the aetiology is considered to be multifactorial (including corticosteroid use, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported particularly in patients with advanced HIV disease and/or long-term exposure to CART. Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Interactions with medicinal products
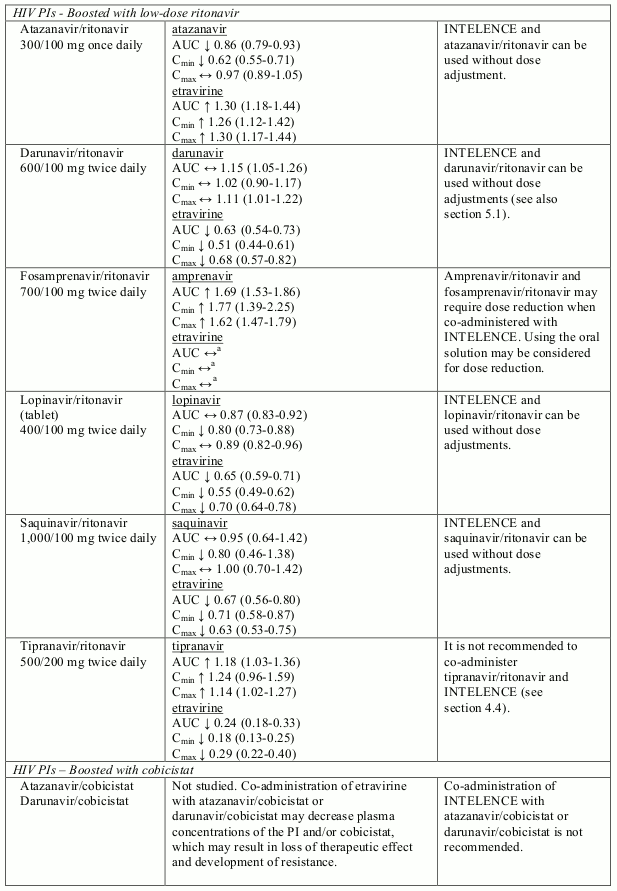
It is not recommended to combine etravirine with tipranavir/ritonavir, due to a marked pharmacokinetic interaction (76% decrease of etravirine AUC) that could significantly impair the virologic response to etravirine.
The combination of etravirine with simeprevir, daclatasvir, atazanavir/cobicistat or darunavir/cobicistat is not recommended (see section 4.5).
For further information on interactions with medicinal products see section 4.5.
Lactose intolerance and lactase deficiency
INTELENCE 25 mg tablets
Each tablet contains 40 mg of lactose monohydrate. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
INTELENCE 100 mg tablets
Each tablet contains 160 mg of lactose monohydrate. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Interaction with other medicinal products and other forms of interaction
Medicinal products that affect etravirine exposure
Etravirine is metabolised by CYP3A4, CYP2C9 and CYP2C19 followed by glucuronidation of the metabolites by uridine diphosphate glucuronosyl transferase (UDPGT). Medicinal products that induce CYP3A4, CYP2C9 or CYP2C19 may increase the clearance of etravirine, resulting in lowered plasma concentrations of etravirine.
Co-administration of etravirine and medicinal products that inhibit CYP3A4, CYP2C9 or CYP2C19 may decrease the clearance of etravirine and may result in increased plasma concentrations of etravirine.
Medicinal products that are affected by the use of etravirine
Etravirine is a weak inducer of CYP3A4. Co-administration of etravirine with medicinal products primarily metabolised by CYP3A4 may result in decreased plasma concentrations of such medicinal products, which could decrease or shorten their therapeutic effects.
Etravirine is a weak inhibitor of CYP2C9 and CYP2C19. Etravirine is also a weak inhibitor of P-glycoprotein. Co-administration with medicinal products primarily metabolised by CYP2C9 or CYP2C19, or transported by P-glycoprotein, may result in increased plasma concentrations of such medicinal products, which could increase or prolong their therapeutic effect or alter their adverse events profile.
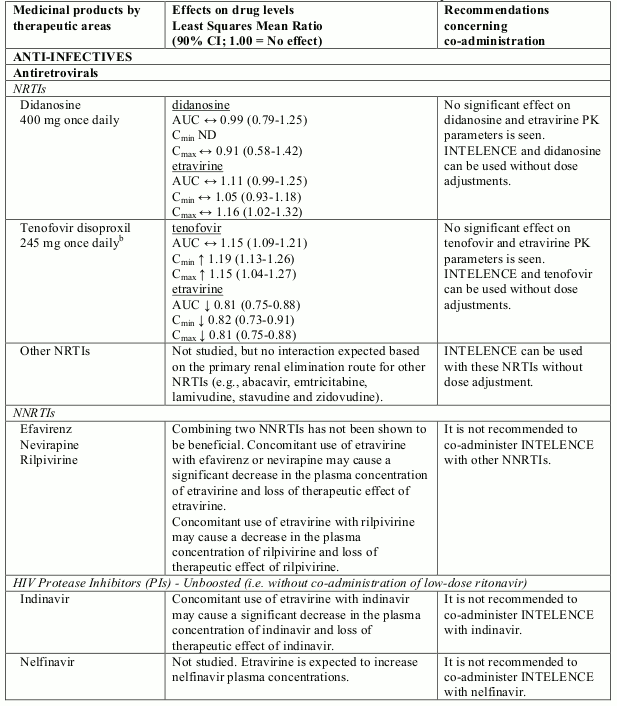
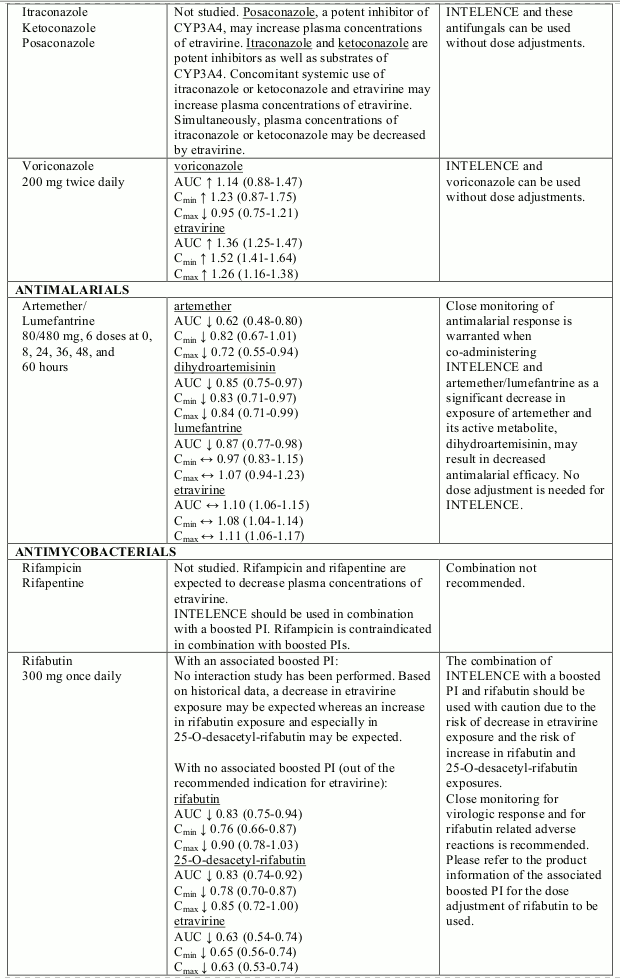
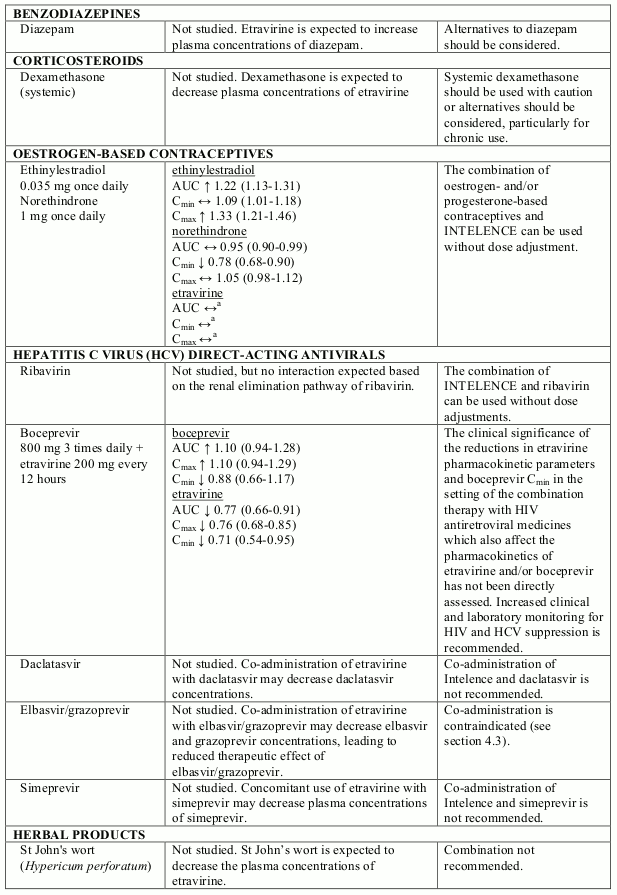
Known and theoretical interactions with selected antiretrovirals and non-antiretroviral medicinal products are listed in table 2. The table is not all-inclusive.
Interaction table
Interactions between etravirine and co-administered medicinal products are listed in table 1 (increase is indicated as “↑”, decrease as “↓”, no change as “↔”, not done as “ND”, confidence interval as “CI”).
Table 2. Interactions and dose recommendations with other medicinal products:
a Comparison based on historic control.
b Study was conducted with tenofovir disoproxil fumarate 300 mg once daily
Note: In drug-drug interaction studies, different formulations and/or doses of etravirine were used which led to similar exposures and, therefore, interactions relevant for one formulation are relevant for the other.
Paediatric population
Interaction studies have only been performed in adults.
Fertility, pregnancy and lactation
Pregnancy
As a general rule, when deciding to use antiretroviral agents for the treatment of HIV infection in pregnant women, and consequently for reducing the risk of HIV vertical transmission to the newborn, the animal data as well as the clinical experience in pregnant women should be taken into account in order to characterise the safety for the foetus.
Placental transfer has been seen in pregnant rats, but it is not known whether placental transfer of etravirine also occurs in pregnant women. Studies in animals do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development, parturition or postnatal development (see section 5.3). Based on animal data the malformative risk is unlikely in humans. The clinical data do not raise safety concern but are very limited.
Breast-feeding
Etravirine is excreted in human milk. Because of the potential for adverse events in nursing infants, women should be instructed not to breastfeed if they are receiving INTELENCE. It is recommended that women living with HIV do not breastfeed in order to avoid transmission of HIV.
Fertility
No human data on the effect of etravirine on fertility are available. In rats, there was no effect on mating or fertility with etravirine treatment (see section 5.3).
Effects on ability to drive and use machines
INTELENCE has minor influence on the ability to drive and use machines. No studies on the effects of INTELENCE on the ability to drive or operate machines have been performed. Adverse reactions such as somnolence and vertigo have been reported in etravirine-treated patients and should be considered when assessing a patient’s ability to drive or operate machinery (see section 4.8).
Undesirable effects
Summary of the safety profile
The most frequent (incidence ≥10%) adverse reactions of all intensities reported for etravirine were rash, diarrhoea, nausea and headache. In the Phase III studies, the rates of discontinuation due to any adverse reaction were 7.2% in patients receiving etravirine. The most common adverse reaction leading to discontinuation was rash.
Tabulated list of adverse reactions
Adverse reactions reported in patients treated with etravirine are summarised in Table 3. The adverse reactions are listed by system organ class (SOC) and frequency. Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10) and uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) and very rare (<1/10,000).
Table 3. Adverse reactions observed with etravirine in clinical trials and post-marketing experience:
| System Organ Class (SOC) | Frequency category | Adverse Reaction |
|---|---|---|
| Blood and lymphatic system disorders | common | thrombocytopaenia, anaemia, decreased neutrophils |
| uncommon | decreased white blood cell count | |
| Immune system disorders | common | drug hypersensitivity |
| uncommon | immune reconstitution syndrome | |
| Metabolism and nutrition disorders | common | diabetes mellitus, hyperglycaemia, hypercholesterolaemia, increased low density lipoprotein (LDL), hypertriglyceridaemia, hyperlipidaemia, dyslipidaemia, anorexia |
| Psychiatric disorders | common | anxiety, insomnia, sleep disorders |
| uncommon | confusional state, disorientation, nightmares, nervousness, abnormal dreams | |
| Nervous system disorders | very common | headache |
| common | peripheral neuropathy, paraesthesia, hypoaesthesia, amnesia, somnolence | |
| uncommon | convulsion, syncope, tremor, hypersomnia, disturbance in attention | |
| Eye disorders | common | blurred vision |
| Ear and labyrinth disorders | uncommon | vertigo |
| Cardiac disorders | common | myocardial infarction |
| uncommon | atrial fibrillation, angina pectoris | |
| Vascular disorders | common | hypertension |
| rare | haemorrhagic strokea | |
| Respiratory, thoracic and mediastinal disorders | common | exertional dyspnoea |
| uncommon | bronchospasm | |
| Gastrointestinal disorders | very common | diarrhoea, nausea |
| common | gastrooesophageal reflux disease, vomiting, abdominal pain, abdominal distension, flatulence, gastritis, constipation, dry mouth, stomatitis, lipase increased, blood amylase increased | |
| uncommon | pancreatitis, haematemesis, retching | |
| Hepatobiliary disorders | common | increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST) |
| uncommon | hepatitis, hepatic steatosis, cytolytic hepatitis, hepatomegaly | |
| Skin and subcutaneous tissue disorders | very common | rash |
| common | night sweats, dry skin, prurigo | |
| uncommon | angioneurotic oedemaa, swelling face, hyperhidrosis | |
| rare | Stevens-Johnson Syndromea, erythema multiformea | |
| very rare | toxic epidermal necrolysisa, DRESSb | |
| Renal and urinary disorders | common | renal failure, blood creatinine increased |
| Reproductive system and breast disorders | uncommon | gynaecomastia |
| General disorders and administration site conditions | common | fatigue |
| uncommon | sluggishness |
a These adverse reactions were observed in other clinical trials than DUET-1 and DUET-2.
b These adverse reactions have been identified through postmarketing experience with etravirine.
Description of selected adverse reactions
Rash
Rash was most frequently mild to moderate, generally macular to maculopapular or erythematous, mostly occurred in the second week of therapy, and was infrequent after week 4. Rash was mostly self-limiting, and generally resolved within 1-2 weeks on continued therapy (see section 4.4). The incidence of rash was higher in women compared to men in the etravirine arm in the DUET trials (rash ≥ grade 2 was reported in 9/60 [15.0%] women versus 51/539 [9.5%] men; discontinuations due to rash were reported in 3/60 [5.0%] women versus 10/539 [1.9%] men) (see section 4.4). There was no gender difference in severity or treatment discontinuation due to rash. The clinical data are limited and an increased risk of cutaneous reactions in patients with a history of NNRTI-associated cutaneous reaction cannot be excluded (see section 4.4).
Metabolic parameters
Weight and levels of blood lipids and glucose may increase during antiretroviral therapy (see section 4.4).
Immune reconstitution syndrome
In HIV infected patients with severe immune deficiency at the time of initiation of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic infections may arise. Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment (see section 4.4).
Osteonecrosis
Cases of osteonecrosis have been reported, particularly in patients with generally acknowledged risk factors, advanced HIV disease or long-term exposure to combination antiretroviral therapy. The frequency of this is unknown (see section 4.4).
Paediatric population (1 year to less than 18 years of age)
The safety assessment in children and adolescents is based on two single-arm trials. PIANO (TMC125-C213) is a Phase II trial in which 101 antiretroviral treatment-experienced HIV-1 infected paediatric patients 6 years to less than 18 years of age received INTELENCE in combination with other antiretroviral agents. TMC125-C234/IMPAACT P1090 is a Phase I/II trial in which 26 antiretroviral treatment-experienced HIV-1 infected paediatric patients aged 1 years to less than 6 years received INTELENCE in combination with other antiretroviral agents (see section 5.1).
In PIANO and TMC125-C234/IMPAACT P1090, the frequency, type and severity of adverse reactions in paediatric patients were comparable to those observed in adults. In PIANO, rash was reported more frequently in female subjects than in male subjects (rash ≥ grade 2 was reported in 13/64 [20.3%] females versus 2/37 [5.4%] males; discontinuations due to rash were reported in 4/64 [6.3%] females versus 0/37 [0%] males) (see section 4.4). Most often, rash was mild to moderate, of macular/papular type, and occurred in the second week of therapy. Rash was mostly self-limiting and generally resolved within 1 week on continued therapy.
In a postmarketing retrospective cohort study aiming at substantiating the long-term safety profile of etravirine in HIV-1-infected children and adolescents receiving etravirine with other HIV-1 antiretrovirals (N=182), Stevens-Johnson Syndrome was reported at a higher incidence (1%) than has been reported in adult clinical trials (<0.1%).
Other special populations
Patients co-infected with hepatitis B and/or hepatitis C virus
In the pooled analysis for DUET-1 and DUET-2, the incidence of hepatic events tended to be higher in co-infected subjects treated with etravirine compared to co-infected subjects in the placebo group. INTELENCE should be used with caution in these patients (see also sections 4.4 and 5.2).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.