ITRACONAZOLE Oral solution Ref.[6946] Active ingredients: Itraconazole
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: UK: Beacon Pharmaceuticals Limited, DCC Vital, Westminster Industrial Estate, Repton Road, Measham, DE12 7DT, England IE: Athlone Laboratories Limited, Ballymurray, Co Roscommon, Ireland
Contraindications
Itraconazole oral solution is contraindicated in patients with a known hypersensitivity to itraconazole or to any of the excipients (See section 6.1).
Co-administration of a number of CYP3A4 substrates is contraindicated with Itraconazole Oral Solution (see sections 4.4 and 4.5).
Itraconazole oral solution should not be administered to patients with evidence of ventricular dysfunction such as congestive heart failure (CHF) or a history of CHF except for the treatment of life-threatening or other serious infections (see section 4.4 Special warnings and precautions).
Itraconazole oral solution should not be used during pregnancy for non-life-threatening indications (see section 4.6 Pregnancy and lactation).
Special warnings and precautions for use
Use in patients with gastro-intestinal motility impairment
When treating patients with severe fungal infections or when administering it as fungal prophylaxis to those with abnormal gastro-intestinal motility, patients should be carefully monitored and where appropriate drug therapeutic monitoring should be considered, where available.
Cross-hypersensitivity
There is no information regarding cross hypersensitivity between itraconazole and other azole antifungal agents. Caution should be used in prescribing Itraconazole Oral Solution to patients with hypersensitivity to other azoles.
Cardiac effects
In a healthy volunteer study with Itraconazole IV, a transient asymptomatic decrease of the left ventricular ejection fraction was observed.
Itraconazole has been shown to have a negative inotropic effect and has been associated with reports of congestive heart failure. Heart failure was more frequently reported among spontaneous reports of 400mg total daily dose than among those of lower total daily doses, suggesting that the risk of heart failure might increase with the total daily dose of itraconazole.
Itraconazole oral solution should not be used in patients with congestive heart failure or with a history of congestive heart failure unless the benefit clearly outweighs the risk. This individual benefit/risk assessment should take into consideration factors such as the severity of the indication, the dose and duration of the treatment, and individual risk factors for congestive heart failure. Such patients should be informed of the signs and symptoms of congestive heart failure, should be treated with caution, and should be monitored for signs and symptoms of congestive heart failure during treatment; if such signs or symptoms do occur during treatment, Itraconazole oral solution should be discontinued.
Caution should be exercised when co-administering itraconazole and calcium channel blockers (see section 4.5. Interaction with other medicinal products).
Hepatic effects
Very rare cases of serious hepatotoxicity, including some cases of fatal acute liver failure, have occurred with the use of Itraconazole. Some of these cases involved patients with no pre-existing liver disease. Some of these cases have been observed within the first month of treatment, including some within the first week. Liver function monitoring should be considered in patients receiving itraconazole treatment. Patients should be instructed to promptly report to their physician signs and symptoms suggestive of hepatitis such as anorexia, nausea, vomiting, fatigue, abdominal pain or dark urine. In these patients treatment should be stopped immediately and liver function testing should be conducted. Most cases of serious hepatotoxicity involved patients who had pre-existing liver disease, were treated for systemic indications, had significant other medical conditions and/or were taking other hepatotoxic drugs. In patients with raised liver enzymes or active liver disease, or who have experienced liver toxicity with other drugs, treatment should not be started unless the expected benefit exceeds the risk of hepatic injury. In patients with impaired hepatic function liver enzyme should be carefully monitored when taking itraconazole.
Use in children
Since clinical data on the use of Itraconazole oral solution in paediatric patients is limited, its use in children is not recommended unless the potential benefit outweighs the potential risks (See section 4.3 Contraindications and section 4.8 Undesirable effects).
Use in elderly
Since clinical data on the use of Itraconazole oral solution in elderly patients is limited, it is advised to use Itraconazole oral solution in these patients only if the potential benefit outweighs the potential risks. In general, it is recommended that the dose selection for an elderly patient should be taken into consideration, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see section 4.4).
Hepatic impairment
Limited data are available on the use of oral itraconazole in patients with hepatic impairment. Caution should be exercised when the drug is administered in this patient population. (See 5.2 Pharmacokinetic properties, Special populations, Hepatic impairment). It is recommended that patients with impaired hepatic function be carefully monitored when taking itraconazole. It is recommended that the prolonged elimination half-life of itraconazole observed in the single oral dose clinical trial with itraconazole capsules in cirrhotic patients be considered when deciding to initiate therapy with other medications metabolized by CYP3A4.
In patients with elevated or abnormal liver enzymes or active liver disease, or who have experienced liver toxicity with other drugs, treatment with Itraconazole is strongly discouraged unless there is a serious or life threatening situation where the expected benefit exceeds the risk. It is recommended that liver function monitoring be done in patients with pre-existing hepatic function abnormalities or those who have experienced liver toxicity with other medications (see section 5.2).
Renal impairment
Limited data are available on the use of oral itraconazole in patients with renal impairment. The exposure of itraconazole may be lower in some patients with renal insufficiency and a wide intersubject variation was observed in these subjects receiving the capsule formulation (see section 5.2). Caution should be exercised when this drug is administered in this patient population and adjusting the dose or switching to an alternative antifungal medication may be considered based on an evaluation of clinical effectiveness.
Prophylaxis in neutropenic patients
In clinical trials diarrhoea was the most frequent adverse event. This disturbance of the gastrointestinal tract may result in impaired absorption and may alter the microbiological flora potentially favouring fungal colonisation. Consideration should be given to discontinuing Itraconazole oral solution in these circumstances.
Treatment of severely neutropenic patients
Itraconazole oral solution as treatment for oral and/or esophageal candidosis was not investigated in severely neutropenic patients. Due to the pharmacokinetic properties (See 5.2 Pharmacokinetic properties), Itraconazole oral solution is not recommended for initiation of treatment in patients at immediate risk of systemic candidosis.
Hearing Loss
Transient or permanent hearing loss has been reported in patients receiving treatment with itraconazole. Several of these reports included concurrent administration of quinidine which is contraindicated (see sections 4.3 and 4.5). The hearing loss usually resolves when treatment is stopped, but can persist in some patients.
Neuropathy
If neuropathy occurs that may be attributable to Itraconazole oral solution, the treatment should be discontinued.
Cross-resistance
In systemic candidosis, if fluconazole-resistant strains of Candida species are suspected, it cannot be assumed that these are sensitive to itraconazole, hence their sensitivity should be tested before the start of itraconazole therapy
Interaction potential
Itraconazole Oral Solution has a potential for clinically important drug interactions (see section 4.5).
Itraconazole should not be used within 2 weeks after discontinuation of treatment with CYP 3A4 inducing agents (rifampicin, rifabutin, phenobarbital, phenytoin, carbamazepine, Hypericum perforatum (St. John’s wort)). The use of itraconazole with these drugs may lead to subtherapeutic plasma levels of itraconazole and thus treatment failure. Co-administration of specific drugs with itraconazole may result in changes in efficacy or safety of itraconazole and/or the co-administered drug. For example, the use of itraconazole with CYP3A4 inducing agents may lead to sub-therapeutic plasma concentrations of itraconazole and thus treatment failure. In addition, the use of itraconazole with some substrates of CYP3A4 can lead to increases in plasma concentrations of these drugs and to serious and/or potentially life threatening adverse events, such as QT prolongation and ventricular tachyarrhythmias including occurrences of torsade de pointes, a potentially fatal arrhythmia. The prescriber should refer to the co-administered medicinal product information for further information regarding serious or life threatening adverse events that could occur in cases of increased plasma concentrations for that medication. For recommendations concerning the co-administration of medicinal products which are contraindicated, not recommended or recommended for use with caution in combination with itraconazole please refer to section 4.5.
Patients with hereditary fructose intolerance (HFI) should not take/be given this medicinal
Product. This medicine contains small amounts of ethanol (alcohol), less than 100mg per dose, 2,080mg propylene glycol per 20 mil solution (maximum single dose) which is equivalent to 103.6 mg/ml and less than 1mmol sodium (23mg) per mil, that is to say essentially “sodium free”.
While propylene glycol has not been shown to cause reproductive or developmental toxicity in animals or humans, it may reach the foetus and was found in milk. As a consequence, administration of propylene glycol to pregnant or lactating patients should be considered on a case by case basis.
Medical monitoring is required in patients with impaired renal or hepatic functions because various adverse events attributed to propylene glycol have been reported such as renal dysfunction (acute tubular necrosis), acute renal failure and liver dysfunction (see section 4.8 Undesirable effects).
Interaction with other medicinal products and other forms of interaction
Itraconazole is mainly metabolized through CYP3A4. Other substances that either share this metabolic pathway or modify CYP3A4 activity may influence the pharmacokinetics of itraconazole. Similarly, itraconazole may modify the pharmacokinetics of other substances that share this metabolic pathway. Itraconazole is a strong CYP3A4 inhibitor and a P-glycoprotein inhibitor. When using concomitant medication, it is recommended that the corresponding label be consulted for information on the route of metabolism and the possible need to adjust dosages.
Drugs that may decrease itraconazole plasma concentrations
Itraconazole is mainly metabolised through the cytochrome CYP3A4.
Coadministration of itraconazole with strong enzyme inducers of CYP3A4 may decrease the exposureofexposureofexposure itraconazole and hydroxy-itraconazole to such an extent that efficacy may be largely reduced.
Antibacterials: isoniazid, rifabutin (see also under ‘Drugs that may have their plasma concentrations increased by itraconazole’), rifampicin.
Anticonvulsants: carbamazepine, (see also under ‘Drugs that may have their plasma concentrations increased by itraconazole’), phenobarbital, phenytoin.
Antivirals: efavirenz, nevirapine.
Herbal medicine: Hypericum perforatum (St John’s Wort).
Therefore, administration of strong enzyme inducers of CYP3A4 with itraconazole is not recommended. It is recommended that the use of these drugs be avoided from 2 weeks before and during treatment with itraconazole, unless the benefits outweigh the risk of potentially reduced itraconazole efficacy. Upon co-administration, it is recommended that the antifungal activity be monitored and the itraconazole dose increased as deemed necessary
Drugs that may increase itraconazole plasma concentrations
Strong inhibitors of CYP3A4 may increase the exposure of itraconazole. Examples include:
Antibacterials: ciprofloxacin, clarithromycin, erythromycin.
Antivirals: ritonavir-boosted darunavir, ritonavir-boosted fosamprenavir, indinavir (see also under ‘Drugs that may have their plasma concentrations increased by itraconazole’), ritonavir (see also under ‘Drugs that may have their plasma concentrations increased by itraconazole’) and telaprevir).
It is recommended that these drugs be used with caution when co-administered with itraconazole oral solution. It is recommended that patients who must take itraconazole concomitantly with strong inhibitors of CYP3A4 be monitored closely for signs or symptoms of increased or prolonged pharmacologic effects of itraconazole, and the itraconazole dose be decreased as deemed necessary. When appropriate, it is recommended that itraconazole plasma concentrations be measured.
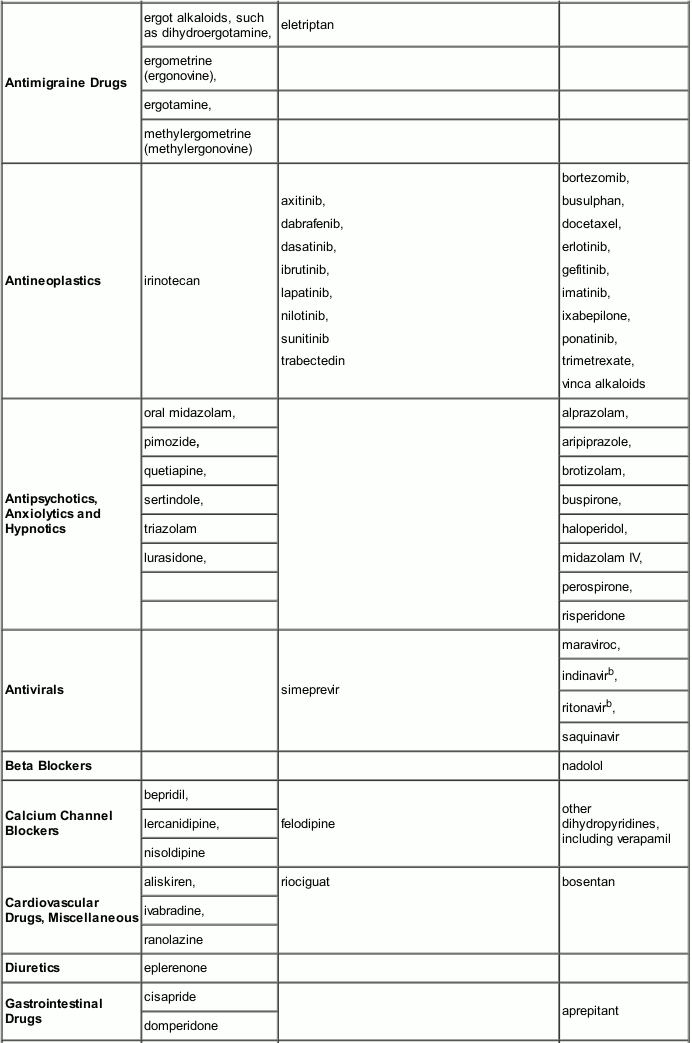
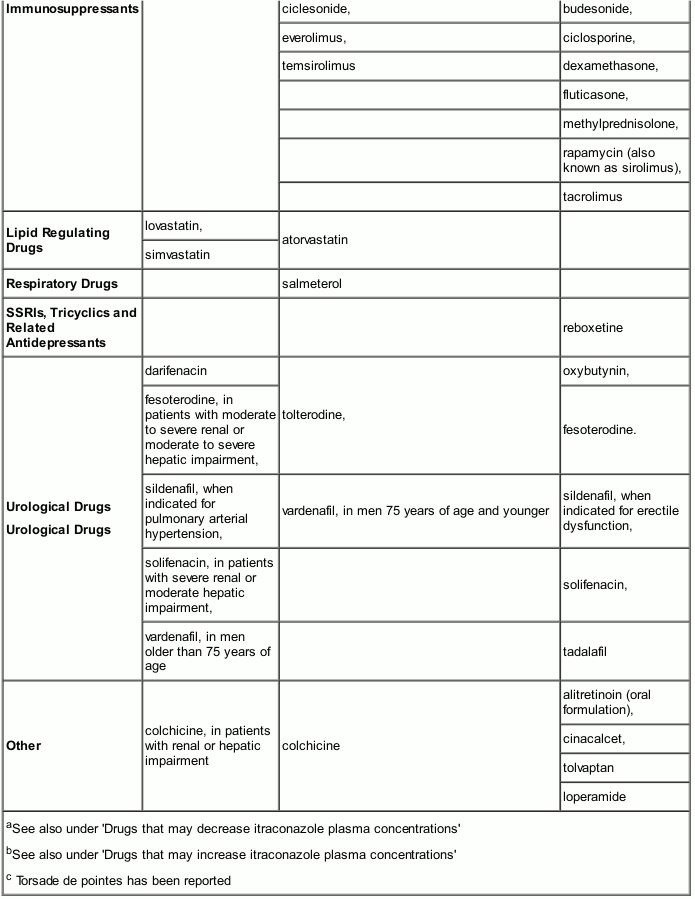
Drugs that may have their plasma concentrations increased by Itraconazole
Itraconazole and its major metabolite, hydroxy-itraconazole can inhibit the metabolism of drugs metabolised by the cytochrome 3A family and can inhibit the drug transport by P-glycoprotein which may result in increased plasma concentrations of these drugs and/or their active metabolite(s) when they are administered with itraconazole. When using concomitant medication, the corresponding label should be consulted for information on the route of metabolism. The effect of itraconazole in increasing the AUC of other drugs can be as high as 11-fold, as seen with oral midazolam (a sensitive CYP3A4 substrate) when co-administered with itraconazole 200mg/d. These elevated plasma concentrations are likely to increase or prolong both therapeutic and adverse effects of these drugs. CYP3A4-metabolized drugs known to prolong the QT interval may be contraindicated with itraconazole, since the combination may lead to ventricular tachyarrhythmias including occurrences of torsade de pointes, a potentially fatal arrhythmia. Full inhibitory effect is not obtained until itraconazole steady state has been reached which takes around 15 days for Itraconazole Oral Solution (see section 5.2). Once treatment is stopped, itraconazole plasma concentrations decrease to an almost undetectable concentration within 7 to 14 days, depending on the dose and duration of treatment. In patients with hepatic cirrhosis or in subjects receiving CYP3A4 inhibitors, the decline in plasma concentrations may be even more gradual. This is particularly important when initiating therapy with drugs whose metabolism is affected by itraconazole.
The interacting drugs are categorized as contraindicated, not recommended or to be used with caution with itraconazole taking into account the extent of the concentration increase and the safety profile of the interacting drug. The interaction potential of the listed drugs was evaluated based on human pharmacokinetic studies with itraconazole, and/or human pharmacokinetic studies with other strong CYP3A4 inhibitors (e.g. ketoconazole) and/or in vitro data:
- ‘Contraindicated’: Under no circumstances is the drug to be co-administered with itraconazole, and up to two weeks after discontinuation of treatment with itraconazole.
- ‘Not recommended’: It is recommended that the use of the drug be avoided during and up to two weeks after discontinuation of treatment with itraconazole, unless the benefits outweigh the potentially increased risks of side effects. If co-administration cannot be avoided, clinical monitoring for signs or symptoms of increased or prolonged effects or side effects of the interacting drug is recommended, and its dosage be reduced or interrupted as deemed necessary. When appropriate, it is recommended that plasma concentrations be measured.
- ‘Use with caution’: Careful monitoring is recommended when the drug is co-administered with itraconazole. Upon co-administration, it is recommended that patients be monitored closely for signs or symptoms of increased or prolonged effects or side effects of the interacting drug, and its dosage be reduced as deemed necessary. When appropriate, it is recommended that plasma concentrations be measured.
Examples of drugs that may have their plasma concentrations increased by itraconazole presented by drug class with advice regarding coadministration with itraconazole:
Caution should be exercised when co-administering itraconazole with calcium channel blockers due to an increased risk of congestive heart failure. In addition to possible pharmacokinetic interactions involving the drug metabolising enzyme CYP3A4, calcium channel blockers can have negative inotropic effects which may be additive to those of itraconazole.
No interaction of itraconazole with zidovudine (AZT) and fluvastatin has been observed.
No inducing effects of itraconazole on the metabolism of ethinyloestradiol and norethisterone were observed.
Effect on protein binding:
In vitro studies have shown that there are no interactions on the plasma protein binding between itraconazole and imipramine, propranolol, diazepam, cimetidine, indometacin, tolbutamide and sulfamethazine.
Drugs that may have their plasma concentrations decreased by itraconazole
Co-administration of itraconazole with the NSAID meloxicam may decrease the plasma concentration of meloxicam. It is recommended that meloxicam be used with caution when co-administered with itraconazole, including monitoring for any reduction in efficacy of meloxicam with adjustments to the dose as necessary.
Paediatric Population
Interaction studies have only been performed in adults.
Fertility, pregnancy and lactation
Pregnancy
Itraconazole oral solution must not be used during pregnancy except for life-threatening cases where the potential benefit to the mother outweighs the potential harm to the foetus (see 4.3 Contraindications).
In animal studies itraconazole has shown reproduction toxicity (see 5.3 Preclinical safety data).
Epidemiological data on exposure to Itraconazole during the first trimester of pregnancy – mostly in patients receiving short-term treatment for vulvovaginal candidosis – did not show an increased risk for malformations as compared to control subjects not exposed to any known teratogens.
Women of child-bearing potential
Women of childbearing potential taking Itraconazole oral solution should use contraceptive precautions. Effective contraception should be continued until the next menstrual period following the end of Itraconazole therapy.
Fertility
In the rat, itraconazole had no effect on male or female fertility at doses which exhibited signs of general toxicity. The effect in humans is unknown.
Lactation
A very small amount of itraconazole is excreted in human milk. Itraconazole Oral Solution must not be used during lactation.
Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed.
When driving vehicles and operating machinery the possibility of adverse reactions such as dizziness, visual disturbances and hearing loss (see Section 4.8 Undesirable effects), which may occur in some instances, must be taken into account.
Undesirable effects
Approximately 9% of patients can be expected to experience adverse reactions while taking itraconazole. In patients receiving prolonged (approximately 1 month) continuous treatment especially, the incidence of adverse events has been higher (about 15%). The most frequently reported adverse experiences have been of gastrointestinal, hepatic and dermatological origin.
The table below presents adverse drug reactions by System Organ Class. Within each System Organ Class, the adverse drug reactions are presented by incidence, using the following convention:
Very common (≥1/10); Common (≥1/100 to <1/10); Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000); Very rare (<1/10,000), Not known (cannot be estimated from the available data).
Blood and lymphatic system disorders
Uncommon: Leucopenia, Neutropenia, Thrombocytopenia
Immune system disorders
Uncommon: Hypersensitivity*
Not Known: Serum Sickness, Angioneurotic Oedema, Anaphylactic Reaction, Anaphylactoid Reaction,
Metabolism and nutrition disorders
Uncommon: Hypokalaemia
Not Known: Hypertriglyceridemia
Nervous system disorders
Common: Headache, Dizziness, Dysgeusia
Uncommon: Peripheral Neuropathy*, Paraesthesia, Hypoaesthesia
Eye disorders
Uncommon: Visual Disorders, including Vision Blurred and Diplopia
Ear and labyrinth disorder
Uncommon: Tinnitus
Not Known: Transient or permanent hearing loss*
Cardiac disorders
Uncommon: Cardiac failure
Not Known: Congestive Heart Failure*
Respiratory, thoracic and mediastinal disorders
Common: Dyspnoea, cough
Not Known: Pulmonary Oedema
Gastrointestinal disorders
Common: Abdominal Pain, Vomiting, Nausea, Diarrhoea, Dyspepsia
Uncommon: Constipation
Not Known: Pancreatitis
Hepato-biliary disorders
Common: Hepatic enzyme increased
Uncommon: Hepatitis, hepatic failure*, Hyperbilirubinaemia
Not Known: Hepatotoxicity* including some cases of fatal Acute hepatic failure*
Skin and subcutaneous tissue disorders
Common: Rash
Uncommon: Urticaria, Pruritus
Not Known: Toxic epidermal necrolysis, Stevens-Johnson syndrome, acute generalised exanthematous pustulosis, erythema multiforme, exfoliative dermatitis, leukocytoclastic vasculitis, alopecia, photosensitivity
Musculoskeletal and connective tissue disorders
Uncommon: Myalgia, arthralgia
Renal and urinary disorders
Not Known: Pollakiuria, urinary incontinence
Reproductive system and breast disorders
Uncommon: Menstrual disorders
Not Known: Erectile dysfunction
General disorders and administration site conditions
Common: Pyrexia
Uncommon: Oedema
Investigations
Not Known: Blood creatine phosphokinase increased
* see section 4.4.
The following is a list of additional ADRs associated with itraconazole that have been reported in clinical trials of Itraconazole Capsules and Itraconazole IV, excluding the ADR term “Injection site inflammation”, which is specific to the injection route of administration.
Infections and infestations: Sinusitis, Upper respiratory tract infection, Rhinitis
Blood and lymphatic system disorders: Granulocytopenia
Metabolism and nutrition disorders: Hyperglycaemia, Hyperkalaemia, Hypomagnesaemia
Psychiatric disorders: Confusional state
Nervous system disorders: Somnolence, Tremor
Cardiac disorders: Left ventricular failure, Tachycardia
Vascular disorders: Hypertension, Hypotension
Respiratory, thoracic and mediastinal disorders: Dysphonia
Gastrointestinal disorders: Gastrointestinal disorder, Flatulence
Hepatobiliary disorders: Hepatic function abnormal
Skin and subcutaneous tissue disorders: Rash erythematous, Hyperhidrosis
Renal and urinary disorders: Renal impairment
General disorders and administration site conditions: Face oedema, Chest pain, Pain, Fatigue, Chills
Investigations: Alanine aminotransferase increased, Aspartate aminotransferase increased, Blood alkaline phosphatase increased, Blood lactate dehydrogenase increased, Blood urea increased, Gamma-glutamyltransferase increased, Hepatic enzyme increased, Urine analysis abnormal
Propylene Glycol: Various adverse events, such as hyperosmolality, lactic acidosis; renal dysfunction (acute tubular necrosis), acute renal failure; cardiotoxicity (arrhythmia, hypotension); central nervous system disorders (depression, coma, seizures); respiratory depression, dyspnoea; liver dysfunction; haemolytic reaction (intravascular haemolysis) and haemoglobinuria; or multisystem organ dysfunction, have been reported with high doses or prolonged use of propylene glycol.
Adverse events usually reverse following weaning off of propylene glycol, and in more severe cases following haemodialysis.
Paediatric Population
The safety of Itraconazole oral solution was evaluated in 250 paediatric patients aged 6 months to 14 years who participated in five open-label clinical trials. These patients received at least one dose of itraconazole for prophylaxis of fungal infections or for treatment of oral thrush or systemic fungal infections and provided safety data.
Based on pooled safety data from these clinical trials, the very common reported ADRs in paediatric patients were Vomiting (36.0%), Pyrexia (30.8%), Diarrhoea (28.4%), Mucosal inflammation (23.2%), Rash (22.8%), Abdominal pain (17.2%), Nausea (15.6%), Hypertension (14.0%), and Cough (11.2%). The nature of ADRs in paediatric patients is similar to that observed in adult subjects, but the incidence is higher in the paediatric patients.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the following:
UK: Yellow Card Scheme at: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google play or Apple App Store.
IE: HPRA Pharmacovigilance, Earlsfort Terrace, IRL – Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.