JOENJA Tablet Ref.[107272] Active ingredients: Leniolisib
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
Leniolisib inhibits PI3K-delta by blocking the active binding site of PI3K-delta. In cell-free isolated enzyme assays, leniolisib was selective for PI3K-delta over PI3K-alpha (28-fold), PI3K-beta (43-fold), and PI3K-gamma (257-fold), as well as the broader kinome. In cell-based assays, leniolisib reduced pAkt pathway activity and inhibited proliferation and activation of B and T cell subsets. Gain-of-function variants in the gene encoding the p110-delta catalytic subunit or loss of function variants in the gene encoding the p85-alpha regulatory subunit each cause hyperactivity of PI3K-delta. Leniolisib inhibits the signalling pathways that lead to increased production of PIP3, hyperactivity of the downstream mTOR/Akt pathway, and to the dysregulation of B and T cells.
12.2. Pharmacodynamics
Ex vivo pharmacodynamics of leniolisib [proportion of phosphorylated Akt (pAkt)-positive B cells] were assessed intra-individually at 10, 30, and 70 mg twice daily for 4 weeks at each dose level in patients with APDS. Within the explored dose range, higher leniolisib plasma concentrations were generally associated with higher reduction of pAkt-positive B cells and higher doses were associated with a slightly higher peak reduction as well as more sustained reduction. Treatment with JOENJA 70 mg twice a day at steady state is estimated to produce time-averaged reduction of pAkt-positive B cells by approximately 80%.
12.3. Pharmacokinetics
The systemic drug exposure (AUC and Cmax) of leniolisib increased dose proportionally within the studied range of doses (20 to 140 mg twice a day dosing and single doses of 10 to 400 mg). During twice daily dosing approximately 12 hours apart, leniolisib accumulates approximately 1.4-fold (range of 1.0 to 2.2) in achieving steady-state, consistent with an effective half-life (t1/2) of approximately 7 hours. Steady state drug concentrations can be expected to be reached after approximately 2 to 3 days of JOENJA treatment. The pharmacokinetics of leniolisib are similar between healthy participants and APDS patients.
Gastric Acid Reducing Agents: Leniolisib exhibits pH-dependent solubility (pH range of 1.2 to 4), with low solubility at higher pH values (≥ 5). However, PK results from APDS patients did not indicate that acid reducing agents (e.g., H2-antagonists, proton pump inhibitors) have a clinically relevant effect on leniolisib systemic exposure.
CYP1A2 Substrates with Narrow Therapeutic Indices: Time-dependent (irreversible) inhibition of CYP1A2 was observed in the presence of leniolisib in vitro.
Transporters: In vitro, leniolisib is a substrate and an inhibitor of the hepatic efflux transporter BCRP and a substrate of P-gp. Leniolisib was identified in vitro as a potential inhibitor of the hepatic uptake and efflux transporters OATP1B1/B3 and BCRP.
Absorption
In a placebo controlled, single and multiple ascending dose study in healthy participants, leniolisib median time to maximum plasma concentration (Tmax) occurred at about 1 hour postdose. Tmax appeared independent of dose and was not altered after multiple oral doses. Food is unlikely to have a clinically meaningful effect on the systemic exposure of leniolisib during JOENJA treatment.
Distribution
The systemic decay in leniolisib plasma concentration over time is bi-exponential, indicating a distribution delay towards peripheral tissues. The apparent terminal elimination t1/2 is approximately 10 hours. The volume of distribution of leniolisib is estimated to be 28.5 L in patients with APDS. Leniolisib was highly bound (94.5%) to plasma proteins.
Elimination
The mean recovery of total 14C-radioactivity following a single oral dose of 70 mg 14C-leniolisib was 92.5% (67.0% and 25.5% recovered via feces and urine, respectively) 168 hours postdose. Unchanged leniolisib (6.32%) was the predominant drug-related material recovered in urine.
Metabolism
Leniolisib was 60% metabolized by the liver, with CYP3A4 being the most predominant enzyme involved (95.4%) in the primary oxidative metabolism of leniolisib with minor contribution from other enzymes (3.5% CYP3A5, 0.7% CYP1A2 and 0.4% CYP2D6). Intestinal secretion by BCRP as well as extrahepatic CYP1A1 cannot be excluded as excretion routes.
Specific Populations
Pediatric Patients
Following a single 70 mg oral dose of leniolisib in APDS patients, leniolisib systemic exposures were comparable between pediatric patients (12 to 17 years of age) and adults (≥ 18 years of age), with median Tmax (ranging from 1 to 5 hours) reached approximately 3 hours post-dose in patients 12 to 17 years of age. The observed difference in the median Tmax between pediatric patients (12 to 17 years of age) and adults (≥ 18 years of age) is not clinically relevant given the PK variability and comparable concentration-time profiles between the two age groups.
Patients with Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of leniolisib has not been evaluated. As leniolisib is metabolized to a large extent by the liver (60%), use of JOENJA is not recommended in patients with moderate to severe hepatic impairment [see Specific Populations (8.6)].
Drug Interaction Studies
Strong CYP3A4 Inhibitors: Leniolisib-exposure was increased approximately 2 fold when administered with itraconazole (strong CYP3A4 inhibitor).
Moderate CYP3A4 Inhibitors: Physiological based pharmacokinetic (PBPK) model-based simulations predicted a maximum increase of 75% in leniolisib AUC0-12 with erythromycin (moderate CYP3A4 inhibitor).
CYP3A4 Inducers: PBPK model-based simulations predicted a maximum decrease of 78% and 58% in leniolisib AUC0-12 with rifampin (strong CYP3A4 inducer) and efavirenz (moderate CYP3A4 inducer), respectively.
CYP2D6 and P-gp inhibitors: Quinidine (strong P-gp and CYP2D6 inhibitor) had no effect on leniolisib systemic exposure. Leniolisib is not a sensitive substrate of P-gp and CYP2D6.
Oral Contraceptives: When combined with a monophasic oral contraceptive containing levonorgestrel and ethinylestradiol, leniolisib increased ethinylestradiol exposure by approximately 25 to 30% in terms of both AUC and Cmax, but did not affect the Cmax or AUC of levonorgestrel. Efficacy of a combined oral contraceptive composed of ethinylestradiol and levonorgestrel is not expected to be compromised by concomitant use with leniolisib.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with leniolisib.
Leniolisib was not genotoxic in the in vitro Ames assay, in vitro chromosomal aberration assay in human lymphocytes, or micronucleus assays in TK6 cells (in vitro) and rats (in vivo).
In a fertility study, male rats had decreased round spermatids and decreased spermatocytes in the testis at an oral dose of 90 mg/kg/day (approximately 2 times the MRHD on an AUC basis). Leniolisib had no effect on fertility in female rats at oral doses up to 90 mg/kg/day (approximately 4 times the MRHD on an AUC basis). No effects on male or female fertility and reproductive performance indices were observed up to the maximum dose administered of 90 mg/kg/day (approximately 2-4 times the MRHD on an AUC basis).
14. Clinical Studies
The efficacy of JOENJA was evaluated in the placebo-controlled portion of Study 2201 (NCT02435173), a 12-week blinded, randomized, placebo-controlled study in adult and pediatric patients 12 years of age and older with confirmed APDS-associated genetic PI3Kδ mutation with a documented variant in either PIK3CD or PIK3R1. Baseline patient demographics are shown in Table 2.
Table 2. Baseline Demographic and Disease Characteristics in Patients with APDS (Study 2201):
| Demographics and Disease Characteristics | JOENJA (N=21) | Placebo (N=10) |
|---|---|---|
| Demographics | ||
| Age1(Years) Mean (SD) | 22.2 (10.00) | 26.7 (13.43) |
| Age Categories < 18, n () (Min, Max) ≥ 18, n () (Min, Max) | 8 (38) (12, 17) 13 (62) (18, 54) | 4 (40) (15, 17) 6 (60) (18, 48) |
| Sex, n (%) Male Female | 11 (52) 10 (48) | 4 (40) 6 (60) |
| Race, n (%) Asian Black White Other | 1 (5) 1 (5) 18 (86) 1 (5) | 1 (10) 1 (10) 7 (70) 1 (10) |
| Ethnicity, n (%) Hispanic or Latino Not Hispanic or Latino Not reported | 0 14 (67) 7 (33) | 1 (10) 7 (70) 2 (20) |
| Disease Characteristics | ||
| APDS 1 (PIK3CD variant), n (%) | 16 (76) | 9 (90) |
| APDS 2 (PIK3R1 variant), n (%) | 5 (24) | 1 (10) |
| Concomitant glucocorticoids, n (%) | 12 (57) | 6 (60) |
| Concomitant immunoglobulin G (IgG), n (%) | 14 (67) | 7 (70) |
| Previous rapamycin/sirolimus use, n (%) | 4 (19) | 3 (30) |
1 Patient age from study Day -4 up to initial JOENJA dosing
Patients had nodal and/or extranodal lymphoproliferation, as measured by index nodal lesion selected by the Cheson methodology on CT or MRI and clinical findings and manifestations compatible with APDS (e.g., history of repeated oto-sino-pulmonary infections, organ dysfunction). Immunosuppressive medications or PI3Kδ inhibitors (selective or non-selective) were prohibited within 6 weeks of baseline (Day -1 and the visit prior to first study drug administration) and throughout the study. In addition, patients who had previous or concurrent B cell depleters (e.g., rituximab) within 6 months of baseline were excluded from the study, unless absolute B lymphocytes in the blood were normal. B cell depleters were prohibited throughout the study.
Thirty-one patients were randomized 2:1 to receive either JOENJA 70 mg (N=21) or placebo (N=10) twice a day for 12 weeks. The co-primary efficacy endpoints were improvement in lymphoproliferation as measured by a change from baseline in lymphadenopathy measured by the log10-transformed sum of product diameters and the normalization of immunophenotype as measured by the percentage of naïve B cells out of total B cells. Both co-primary efficacy endpoints were statistically significant (Table 3).
Table 3. Co-primary Endpoints in Placebo-Controlled Portion of Study 2201 at Week 12 (Day 85):
| JOENJA (N=21) | Placebo (N=10) | |
|---|---|---|
| Log10-Transformed SPD of Index Lesions (Excluding Patients with 0 Lesions at Baseline) a | ||
| n b | 18 | 8 |
| Baseline Mean (SD) | 3.03 (0.42) | 3.05 (0.39) |
| Change from Baseline, LS Mean (SE) | -0.27 (0.04) | -0.02 (0.05) |
| Difference vs. Placebo (95% CI) | -0.25 (-0.38, -0.12) | |
| p-value | 0.0006 | |
| Percentage of Naïve B Cells out of Total B Cells (Patients with < 48% of Naïve B Cells at Baseline) c | ||
| n d | 8 | 5 |
| Baseline e Mean (SD) | 27.16 (13.16) | 30.51 (7.97) |
| Change from Baseline, LS Mean (SE) | 37.39 (5.34) | 0.09 (6.66) |
| Difference vs. Placebo (95% CI) | 37.30 (24.06, 50.54) | |
| p-value | 0.0002 | |
CI=confidence interval; SD= Standard deviation; SE=standard error; SPD=sum of product diameters; vs=versus; LS Mean: Least-squares mean
Note: The LS mean change from baseline, difference in LS mean change from baseline between JOENJA and placebo and its p-value were obtained from an Analysis of Covariance model with treatment, glucocorticoids use and immunoglobulin replacement therapy at baseline, and baseline measurement as covariates.
a Change in index lesion size was measured using the log10 transformed sum of the product of diameters (SPD) of the largest lymph nodes (maximum of 6) identified as per the Cheson criteria on CT/MRI.
b The analysis excluded 2 patients from each treatment group due to protocol deviations and 1 JOENJA patient having complete resolution of the index lesion identified at baseline.
c Cell surface markers used to distinguish naïve B cells on flow cytometry were CD19+ CD27- CD10-.
d The analysis excluded 2 patients from each treatment group due to protocol deviations, 5 JOENJA patients and 3 placebo patients with more than or equal to 48% naïve B cells at baseline, 5 JOENJA patients with no Day 85 measurement, and 1 JOENJA patient with no baseline measurement.
e Baseline is defined as the arithmetic mean of the Baseline and Day 1 values when both were available, and if either value was missing, the existing value was used.
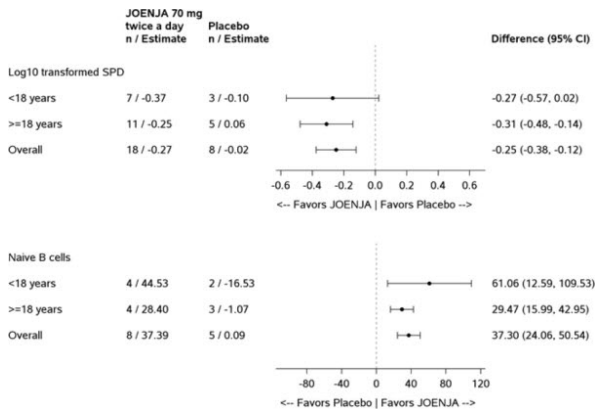
Figure 1 represents the co-primary endpoints grouped by age (< 18 years of age vs ≥ 18 years of age).
Figure 1. Difference from Baseline in Log10 Transformed SPD of Index Lesions and Percentage of Naïve B Cells out of Total B Cells (Patients with < 48% of Naïve B Cells at Baseline) by Age Group:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.