JUBBONTI Solution for injection Ref.[109547] Active ingredients: Denosumab
Source: FDA, National Drug Code (US) Revision Year: 2024
1. Indications and Usage
1.1 Treatment of Postmenopausal Women with Osteoporosis at High Risk for Fracture
Jubbonti is indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy. In postmenopausal women with osteoporosis, Jubbonti reduces the incidence of vertebral, nonvertebral, and hip fractures [see Clinical Studies (14.1)].
1.2 Treatment to Increase Bone Mass in Men with Osteoporosis
Jubbonti is indicated for treatment to increase bone mass in men with osteoporosis at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy [see Clinical Studies (14.2)].
1.3 Treatment of Glucocorticoid-Induced Osteoporosis
Jubbonti is indicated for the treatment of glucocorticoid-induced osteoporosis in men and women at high risk of fracture who are either initiating or continuing systemic glucocorticoids in a daily dosage equivalent to 7.5 mg or greater of prednisone and expected to remain on glucocorticoids for at least 6 months. High risk of fracture is defined as a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy [see Clinical Studies (14.3)].
1.4 Treatment of Bone Loss in Men Receiving Androgen Deprivation Therapy for Prostate Cancer
Jubbonti is indicated as a treatment to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer. In these patients denosumab also reduced the incidence of vertebral fractures [see Clinical Studies (14.4)].
1.5 Treatment of Bone Loss in Women Receiving Adjuvant Aromatase Inhibitor Therapy for Breast Cancer
Jubbonti is indicated as a treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer [see Clinical Studies (14.5)]
2. Dosage and Administration
2.1 Information Essential to Safe Dosing or Administration
Pregnancy must be ruled out prior to administration of Jubbonti. Perform pregnancy testing in all females of reproductive potential prior to administration of Jubbonti. Based on findings in animals, denosumab products can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1, 8.3)].
2.2 Laboratory Testing in Patients with Advanced Chronic Kidney Disease Prior to Initiation of Jubbonti
In patients with advanced chronic kidney disease [i.e., estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m² ], including dialysis-dependent patients, evaluate for the presence of chronic kidney disease mineral and bone disorder (CKD-MBD) with intact parathyroid hormone (iPTH), serum calcium, 25(OH) vitamin D, and 1,25 (OH)2 vitamin D prior to decisions regarding Jubbonti treatment. Consider also assessing bone turnover status (serum markers of bone turnover or bone biopsy) to evaluate the underlying bone disease that may be present [see Warnings and Precautions (5.1)].
2.3 Recommended Dosage
Jubbonti should be administered by a healthcare professional.
The recommended dose of Jubbonti is 60 mg administered as a single subcutaneous injection once every 6 months. Administer Jubbonti via subcutaneous injection in the upper arm, the upper thigh, or the abdomen. All patients should receive calcium 1000 mg daily and at least 400 IU vitamin D daily [see Warnings and Precautions (5.1)].
If a dose of Jubbonti is missed, administer the injection as soon as the patient is available. Thereafter, schedule injections every 6 months from the date of the last injection.
2.4 Preparation and Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Jubbonti is a clear to slightly opalescent and colorless to slightly yellowish to slightly brownish solution. Do not use if the solution is discolored or cloudy or if the solution contains visible particles or foreign particulate matter.
Prior to administration, Jubbonti may be removed from the refrigerator and brought to room temperature (up to 25°C/77°F) by standing in the original container. This generally takes 15 to 30 minutes. Do not warm Jubbonti in any other way [see How Supplied/Storage and Handling (16)].
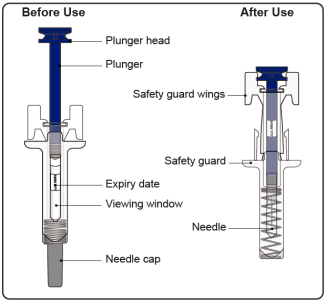
Instructions for Administration of Prefilled Syringe with Needle Safety Guard
- The prefilled syringe has a safety guard that activates to cover the needle after the injection is finished. The safety guard helps to prevent needle stick injuries to anyone who handles the prefilled syringe after injection.
- Do not touch the safety guard wings before use. Touching them may cause the safety guard to activate too early.
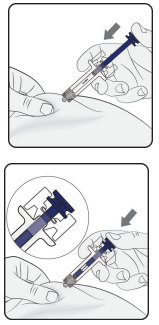
Step 1: Remove Needle Cap
Step 2: Administer Subcutaneous Injection
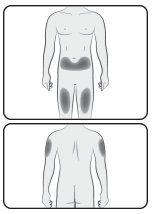
Choose an injection site
The recommended injection sites for Jubbonti include: upper arm, upper thigh, or the abdomen.
Do not press the plunger while inserting the needle.
Start injection
Pinch the skin and insert the needle. Slowly press the plunger as far as it will go. This will ensure that a full dose is injected.
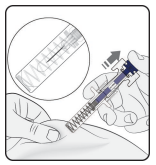
Complete injection
Confirm that the plunger head is between the safety guard wings as shown. This will ensure that the safety guard has been activated and will cover the needle after the injection is finished.
Release plunger
Keeping the prefilled syringe at the injection site, slowly release the plunger until the needle is covered by the safety guard. Remove the prefilled syringe from the injection site and release the pinch.
Do not rub the injection site.
Immediately dispose of the syringe and needle cap in the nearest sharps container. Do not put the needle cap back on the used syringe.
10. Overdosage
There is no experience with overdosage with denosumab products.
16.2. Storage and Handling
Store Jubbonti in a refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton. Do not freeze. Prior to administration, Jubbonti may be allowed to reach room temperature (up to 25°C/77°F) in the original container.
Once removed from the refrigerator, Jubbonti must not be exposed to temperatures above 25°C (77°F) and must be used within 30 days. If not used within the 30 days, Jubbonti should be discarded. Do not use Jubbonti after the expiry date printed on the label.
Protect Jubbonti from direct light and heat.
Avoid vigorous shaking of Jubbonti.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.