KEYTRUDA Concentrate for solution for infusion Ref.[7435] Active ingredients: Pembrolizumab
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, PD-1/PDL-1 (Programmed cell death protein 1/death ligand 1) inhibitors
ATC code: L01FF02
Mechanism of action
KEYTRUDA is a humanised monoclonal antibody which binds to the programmed cell death-1 (PD-1) receptor and blocks its interaction with ligands PD-L1 and PD-L2. The PD-1 receptor is a negative regulator of T-cell activity that has been shown to be involved in the control of T-cell immune responses. KEYTRUDA potentiates T-cell responses, including anti-tumour responses, through blockade of PD-1 binding to PD-L1 and PD-L2, which are expressed in antigen presenting cells and may be expressed by tumours or other cells in the tumour microenvironment.
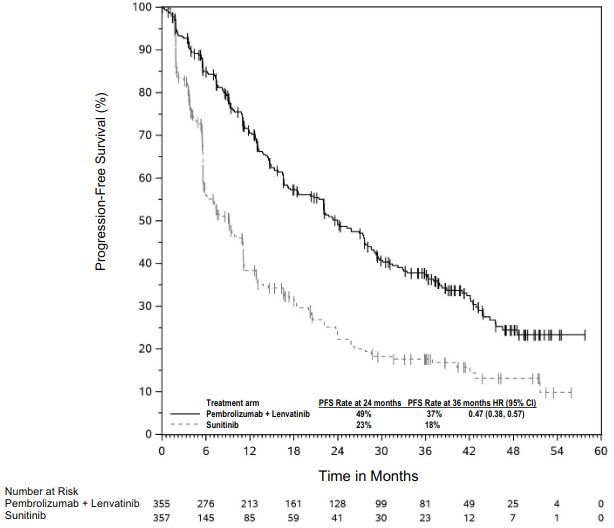
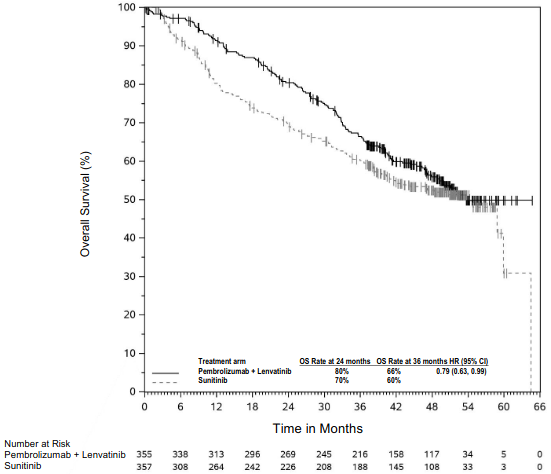
The anti-angiogenic effect of lenvatinib (multi-TKI) in combination with the immune-stimulatory effect of pembrolizumab (anti-PD-1) results in a tumour microenvironment with greater T-cell activation to help overcome primary and acquired resistance to immunotherapy and may improve tumour responses compared to either treatment alone. In preclinical murine models, PD-1 plus TKI inhibitors have demonstrated enhanced anti-tumour activity compared to either agent alone.
Clinical efficacy and safety
Pembrolizumab doses of 2 mg/kg bw every 3 weeks, 10 mg/kg bw every 3 weeks, and 10 mg/kg bw every 2 weeks were evaluated in melanoma or previously treated NSCLC clinical studies. Based on the modelling and simulation of dose/exposure relationships for efficacy and safety for pembrolizumab, there are no clinically significant differences in efficacy or safety among the doses of 200 mg every 3 weeks, 2 mg/kg bw every 3 weeks, and 400 mg every 6 weeks (see section 4.2).
Melanoma
KEYNOTE-006: Controlled study in melanoma patients naïve to treatment with ipilimumab
The safety and efficacy of pembrolizumab were investigated in KEYNOTE-006, a multicentre, open-label, controlled, Phase III study for the treatment of advanced melanoma in patients who were naïve to ipilimumab. Patients were randomised (1:1:1) to receive pembrolizumab 10 mg/kg bw every 2 (n=279) or 3 weeks (n=277) or ipilimumab 3 mg/kg bw every 3 weeks (n=278). Patients with BRAF V600E mutant melanoma were not required to have received prior BRAF inhibitor therapy.
Patients were treated with pembrolizumab until disease progression or unacceptable toxicity. Clinically stable patients with initial evidence of disease progression were permitted to remain on treatment until disease progression was con firmed. Assessment of tumour status was performed at 12 weeks, then every 6 weeks through Week 48, followed by every 12 weeks thereafter.
Of the 834 patients, 60% were male, 44% were ≥65 years (median age was 62 years [range: 18-89]) and 98% were white. Sixty-five percent of patients had M1c stage, 9% had a history of brain metastases, 66% had no and 34% had one prior therapy. Thirty -one percent had an ECOG Performance Status of 1, 69% had ECOG Performance Status of 0 and 32% had elevated LDH. BRAF mutations were reported in 302 (36%) patients. Among patients with BRAF mutant tumours, 139 (46%) were previously treated with a BRAF inhibitor.
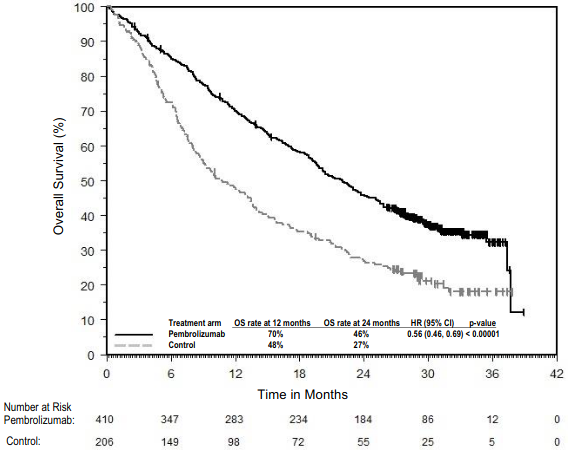
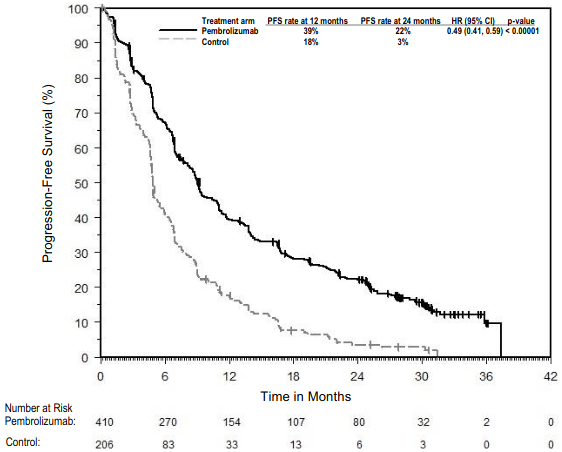
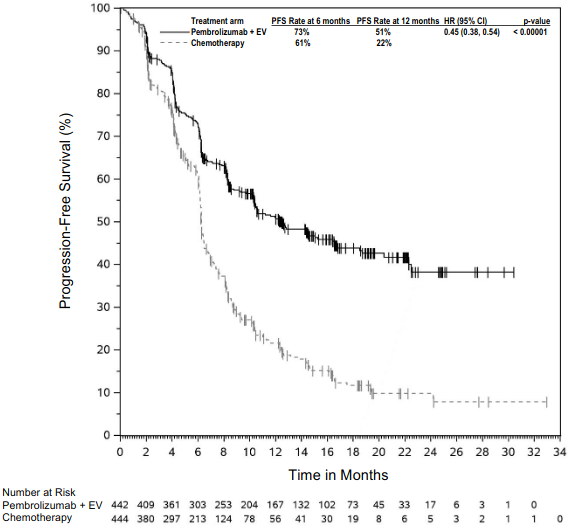
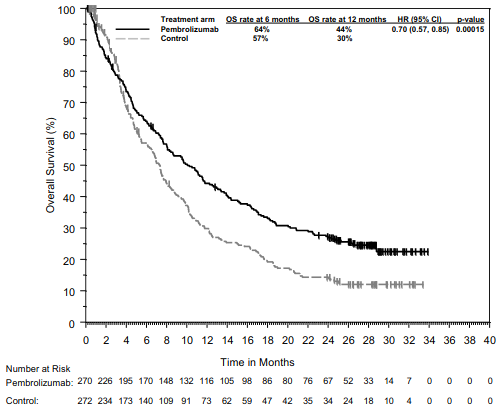
The primary efficacy outcome measures were progression -free survival (PFS; as assessed by Integrated Radiology and Oncology Assessment [IRO] review using Response Evaluation Criteria in Solid Tumours [RECIST], version 1.1) and overall survival (OS). Secondary efficacy outcome measures were objective response rate (ORR) and response duration. Table 3 summarises key efficacy measures in patients naïve to treatment with ipilimumab at the final analysis performed after a minimum of 21 months of follow-up. Kaplan-Meier curves for OS and PFS based on the final analysis are shown in Figures 1 and 2.
Table 3. Efficacy results in KEYNOTE-006:
| Endpoint | Pembrolizumab 10 mg/kg bw every 3 weeks n=277 | Pembrolizumab 10 mg/kg bw every 2 weeks n=279 | Ipilimumab 3 mg/kg bw every 3 weeks n=278 |
|---|---|---|---|
| OS | |||
| Number (%) of patients with event | 119 (43%) | 122 (44%) | 142 (51%) |
| Hazard ratio* (95% CI) | 0.68 (0.53, 0.86) | 0.68 (0.53, 0.87) | --- |
| p-Value† | <0.001 | <0.001 | --- |
| Median in months (95% CI) | Not reached (24, NA) | Not reached (22, NA) | 16 (14, 22) |
| PFS | |||
| Number (%) of patients with event | 183 (66%) | 181 (65%) | 202 (73%) |
| Hazard ratio* (95% CI) | 0.61 (0.50, 0.75) | 0.61 (0.50, 0.75) | --- |
| p-Value† | <0.001 | <0.001 | --- |
| Median in months (95% CI) | 4.1 (2.9, 7.2) | 5.6 (3.4, 8.2) | 2.8 (2.8, 2.9) |
| Best objective response | |||
| ORR % (95% CI) | 36% (30, 42) | 37% (31, 43) | 13% (10, 18) |
| Complete response | 13% | 12% | 5% |
| Partial response | 23% | 25% | 8% |
| Response duration‡ | |||
| Median in months (range) | Not reached (2.0, 22.8+) | Not reached (1.8, 22.8+) | Not reached (1.1+, 23.8+) |
| % ongoing at 18 months | 68%§ | 71%§ | 70%§ |
* Hazard ratio (pembrolizumab compared to ipilimumab) based on the stratified Cox proportional hazard model
† Based on stratified log-rank test
‡ Based on patients with a best objective response as confirmed complete or partial response
§ Based on Kaplan-Meier estimation
NA = not available
Figure 1. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-006 (intent to treat population):
Figure 2. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-006 (intent to treat population):
KEYNOTE-002: Controlled study in melanoma patients previously treated with ipilimumab
The safety and efficacy of pembrolizumab were investigated in KEYNOTE-002, a multicentre, double-blind, controlled study for the treatment of advanced melanoma in patients previously treated with ipilimumab and if BRAF V600 mutation-positive, with a BRAF or MEK inhibitor. Patients were randomised (1:1:1) to receive pembroli zumab at a dose of 2 (n=180) or 10 mg/kg bw (n=181) every 3 weeks or chemotherapy (n=179; including dacarbazine, temozolomide, carboplatin, paclitaxel, or carboplatin+paclitaxel). The study excluded patients with autoimmune disease or those receiving immunosuppression; further exclusion criteria were a history of severe or life-threatening immune-mediated adverse reactions from treatment with ipilimumab, defined as any Grade 4 toxicity or Grade 3 toxicity requiring corticosteroid treatment (>10 mg/day prednisone or equivalent dose) for greater than 12 weeks; ongoing adverse reactions ≥ Grade 2 from previous treatment with ipilimumab; previous severe hypersensitivity to other monoclonal antibodies; a history of pneumonitis or interstitial lung disease; HIV, hepatitis B or hepatitis C infection and ECOG Performance Status ≥2.
Patients were treated with pembrolizumab until disease progression or unacceptable toxicity. Clinically stable patients with initial evidence of disease progression were permitted to remain on treatment until disease progression was confirmed. Assessment of tumour status was performed at 12 weeks, then every 6 weeks through Week 48, followed by every 12 weeks thereafter. Patients on chemotherapy who experienced independently-verified progression of disease after the first scheduled disease assessment were able to crossover and receive 2 mg/kg bw or 10 mg/kg bw of pembrolizumab every 3 weeks in a double-blind fashion.
Of the 540 patients, 61% were male, 43% were ≥65 years (median age was 62 years [range: 15-89]) and 98% were white. Eighty-two percent had M1c stage, 73% had at least two and 32% of patients had three or more prior systemic therapies for advanced melanoma. Forty-five percent had an ECOG Performance Status of 1, 40% had elevated LDH and 23% had a BRAF mutated tumour.
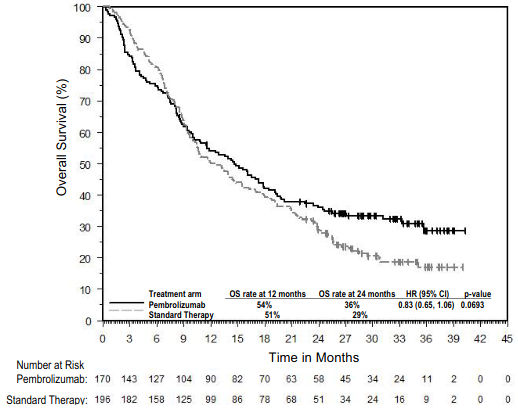
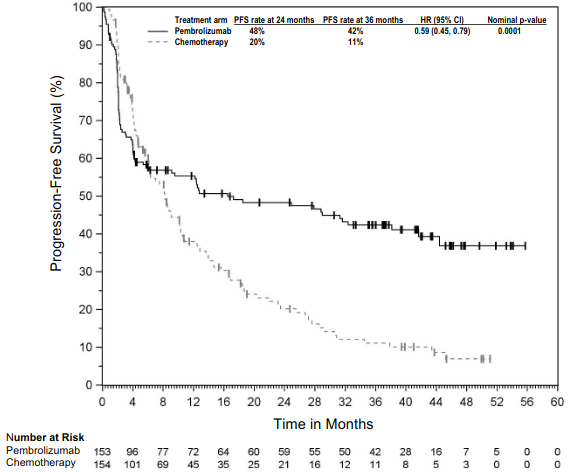
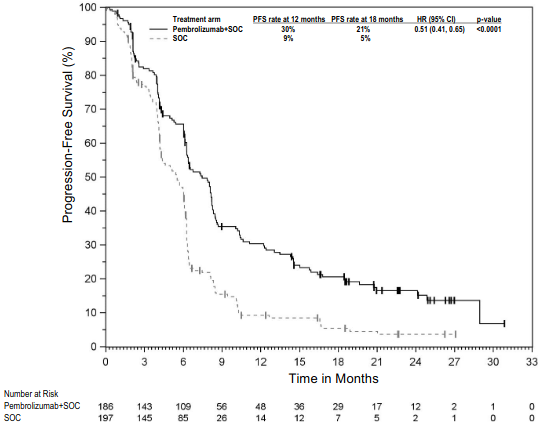
The primary efficacy outcome measures were PFS as assessed by IRO using RECIST version 1.1 and OS. Secondary efficacy outcome measures were ORR and response duration. Table 4 summarises key efficacy measures at the final analysis in patients previously treated with ipilimumab, and the Kaplan-Meier curve for PFS is shown in Figure 3. Both pembrolizumab arms were superior to chemotherapy for PFS, and there was no difference between pembrolizumab doses. There was no statistically significant difference between pembrolizumab and chemotherapy in the final OS analysis that was not adjusted for the potentially confounding effects of crossover. Of the patients randomised to the chemotherapy arm, 55% crossed over and subsequently received treatment with pembrolizumab.
Table 4. Efficacy results in KEYNOTE-002:
| Endpoint | Pembrolizumab 2 mg/kg bw every 3 weeks n=180 | Pembrolizumab 10 mg/kg bw every 3 weeks n=181 | Chemotherapy n=179 |
|---|---|---|---|
| PFS | |||
| Number (%) of patients with event | 150 (83%) | 144 (80%) | 172 (96%) |
| Hazard ratio* (95% CI) | 0.58 (0.46, 0.73) | 0.47 (0.37, 0.60) | --- |
| p-Value† | <0.001 | <0.001 | --- |
| Median in months (95% CI) | 2.9 (2.8, 3.8) | 3.0 (2.8, 5.2) | 2.8 (2.6, 2.8) |
| OS | |||
| Number (%) of patients with event | 123 (68%) | 117 (65%) | 128 (72%) |
| Hazard ratio* (95% CI) | 0.86 (0.67, 1.10) | 0.74 (0.57, 0.96) | --- |
| p-Value† | 0.1173 | 0.0106‡ | --- |
| Median in months (95% CI) | 13.4 (11.0, 16.4) | 14.7 (11.3, 19.5) | 11.0 (8.9, 13.8) |
| Best objective response | |||
| ORR % (95% CI) | 22% (16, 29) | 28% (21, 35) | 5% (2, 9) |
| Complete response | 3% | 7% | 0% |
| Partial response | 19% | 20% | 5% |
| Response duration§ | |||
| Median in months (range) | 22.8 (1.4+, 25.3+) | Not reached (1.1+, 28.3+) | 6.8 (2.8, 11.3) |
| % ongoing at 12 months | 73%¶ | 79%¶ | 0%¶ |
* Hazard ratio (pembrolizumab compared to chemotherapy) based on the stratified Cox proportional hazard model
† Based on stratified log-rank test
‡ Not statistically significant after adjustment for multiplicity
§ Based on patients with a best objective response as confirmed complete or partial response from the final analysis
¶ Based on Kaplan-Meier estimation
Figure 3. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-002 (intent to treat population):
KEYNOTE-001: Open-label study in melanoma patients naïve and previously treated with ipilimumab
The safety and efficacy of pembrolizumab for patients with advanced melanoma were investigated in an uncontrolled, open-label study, KEYNOTE-001. Efficacy was evaluated for 276 patients from two defined cohorts, one which included patients previously treated with ipilimumab (and if BRAF V600 mutation-positive, with a BRAF or MEK inhibitor) and the other which included patients naïve to treatment with ipilimumab. Patients were randomly assigned to receive pembrolizumab at a dose of 2 mg/kg bw every 3 weeks or 10 mg/kg bw every 3 weeks. Patients were treated with pembrolizumab until disease progression or unacceptable toxicity. Clinically stable patients with initial evidence of disease progression were permitted to remain on treatment until disease progression was confirmed. Exclusion criteria were similar to those of KEYNOTE-002.
Of the 89 patients receiving 2 mg/kg bw of pembrolizumab who were previously treated with ipilimumab, 53% were male, 33% were ≥65 years of age and the median age was 59 years (range: 18-88). All but two patients were white. Eighty-four percent had M1c stage and 8% of patients had a history of brain metastases. Seventy percent had at least two and 35% of pa tients had three or more prior systemic therapies for advanced melanoma. BRAF mutations were reported in 13% of the study population. All patients with BRAF mutant tumours were previously treated with a BRAF inhibitor.
Of the 51 patients receiving 2 mg/kg bw of pembrolizumab who were naïve to treatment with ipilimumab, 63% were male, 35% were ≥65 years of age and the median age was 60 years (range: 35-80). All but one patient was white. Sixty-three percent had M1c stage and 2% of patients had a history of brain metastases. Forty-five percent had no prior therapies for advanced melanoma. BRAF mutations were reported in 20 (39%) patients. Among patients with BRAF mutant tumours, 10 (50%) were previously treated with a BRAF inhibitor.
The primary efficacy outcome measure was ORR as assessed by independent review using RECIST 1.1. Secondary efficacy outcome measures were disease control rate (DCR; including complete response, partial response and stable disease), response duration, PFS and OS. Tumour response was assessed at 12 week intervals. Table 5 summarises key efficacy measures in patients previously treated or naïve to treatment with ipilimumab, receiving pembrolizumab at a dose of 2 mg/kg bw based on a minimum follow-up time of 30 months for all patients.
Table 5. Efficacy results in KEYNOTE-001:
| Endpoint | Pembrolizumab 2 mg/kg bw every 3 weeks in patients previously treated with ipilimumab n=89 | Pembrolizumab 2 mg/kg bw every 3 weeks in patients naïve to treatment with ipilimumab n=51 |
|---|---|---|
| Best objective response* by IRO† | ||
| ORR % (95% CI) | 26% (17, 36) | 35% (22, 50) |
| Complete response | 7% | 12% |
| Partial response | 19% | 24% |
| Disease control rate %‡ | 48% | 49% |
| Response duration§ | ||
| Median in months (range) | 30.5 (2.8+, 30.6+) | 27.4 (1.6+, 31.8+) |
| % ongoing at 24 months¶ | 75% | 71% |
| PFS | ||
| Median in months (95% CI) | 4.9 (2.8, 8.3) | 4.7 (2.8, 13.8) |
| PFS rate at 12 months | 34% | 38% |
| OS | ||
| Median in months (95% CI) | 18.9 (11, not available) | 28.0 (14, not available) |
| OS rate at 24 months | 44% | 56% |
* Includes patients without measurable disease at baseline by independent radiology
† IRO = Integrated radiology and oncologist assessment using RECIST 1.1
‡ Based on best response of stable disease or better
§ Based on patients with a confirmed response by independent review, starting from the date the response was first recorded; n=23 for patients previously treated with ipilimumab; n=18 for patients naïve to treatment with ipilimumab
¶ Based on Kaplan-Meier estimation
Results for patients previously treated with ipilimumab (n=84) and naïve to treatment with ipilimumab (n=52) who received 10 mg/kg bw of pembrolizumab every 3 weeks were similar to those seen in patients who received 2 mg/kg bw of pembrolizumab every 3 weeks.
Sub-population analyses
BRAF mutation status in melanoma
A subgroup analysis was performed as part of the final analysis of KEYNOTE-002 in patients who were BRAF wild type (n=414; 77%) or BRAF mutant with prior BRAF treatment (n=126; 23%) as summarised in Table 6.
Table 6. Efficacy results by BRAF mutation status in KEYNOTE-002:
| BRAF wild type | BRAF mutant with prior BRAF treatment | |||
|---|---|---|---|---|
| Endpoint | Pembrolizumab 2 mg/kg bw every 3 weeks (n=136) | Chemotherapy (n=137) | Pembrolizumab 2 mg/kg bw every 3 weeks (n=44) | Chemotherapy (n=42) |
| PFS Hazard ratio* (95% CI) | 0.50 (0.39, 0.66) | --- | 0.79 (0.50, 1.25) | --- |
| OS Hazard ratio* (95% CI) | 0.78 (0.58, 1.04) | --- | 1.07 (0.64, 1.78) | --- |
| ORR % | 26% | 6% | 9% | 0% |
* Hazard ratio (pembrolizumab compared to chemotherapy) based on the stratified Cox proportional hazard model
A subgroup analysis was performed as part of the final analysis of KEYNOTE-006 in patients who were BRAF wild type (n=525; 63%), BRAF mutant without prior BRAF treatment (n=163; 20%) and BRAF mutant with prior BRAF treatment (n=139; 17%) as summarised in Table 7.
Table 7. Efficacy results by BRAF mutation status in KEYNOTE-006:
| BRAF wild type | BRAF mutant with prior BRAF treatment | BRAF mutant with prior BRAF treatment | ||||
|---|---|---|---|---|---|---|
| Endpoint | Pembrolizumab 10 mg/kg bw every 2 or 3 weeks (pooled) | Ipilimumab (n=170) | Pembrolizumab 10 mg/kg bw every 2 or 3 weeks (pooled) | Ipilimumab (n=55) | Pembrolizumab 10 mg/kg bw every 2 or 3 weeks (pooled) | Ipilimumab (n=52) |
| PFS Hazard ratio* (95% CI) | 0.61 (0.49, 0.76) | --- | 0.52 (0.35, 0.78) | --- | 0.76 (0.51, 1.14) | --- |
| OS Hazard ratio* (95% CI) | 0.68 (0.52, 0.88) | --- | 0.70 (0.40, 1.22) | --- | 0.66 (0.41, 1.04) | --- |
| ORR % | 38% | 14% | 41% | 15% | 24% | 10% |
* Hazard ratio (pembrolizumab compared to ipilimumab) based on the stratified Cox proportional hazard model
PD-L1 status in melanoma
A subgroup analysis was performed as part of the final analysis of KEYNOTE-002 in patients who were PD-L1 positive (PD -L1 expression in ≥1% of tumour and tumour-associated immune cells relative to all viable tumour cells – MEL score) vs. PD-L1 negative. PD-L1 expression was tested retrospectively by immunohistochemistry (IHC) assay with the 22C3 anti -PD-L1 antibody. Among patients who were evaluable for PD-L1 expression (79%), 69% (n=294) were PD-L1 positive and 31% (n=134) were PD-L1 negative. Table 8 summarises efficacy results by PD -L1 expression.
Table 8. Efficacy results by PD-L1 expression in KEYNOTE-002:
| Endpoint | Pembrolizumab 2 mg/kg bw every 3 weeks | Chemotherapy | Pembrolizumab 2 mg/kg bw every 3 weeks | Chemotherapy |
|---|---|---|---|---|
| PD-L1 positive | PD-L1 negative | |||
| PFS Hazard ratio* (95% CI) | 0.55 (0.40, 0.76) | --- | 0.81 (0.50, 1.31) | --- |
| OS Hazard ratio* (95% CI) | 0.90 (0.63, 1.28) | --- | 1.18 (0.70, 1.99) | --- |
| ORR % | 25% | 4% | 10% | 8% |
* Hazard ratio (pembrolizumab compared to chemotherapy) based on the stratified Cox proportional hazard model
A subgroup analysis was performed as part of the final analysis of KEYNOTE-006 in patients who were PD-L1 positive (n=671; 80%) vs. PD-L1 negative (n=150; 18%). Among patients who were evaluable for PD-L1 expression (98%), 82% were PD-L1 positive and 18% were PD-L1 negative. Table 9 summarises efficacy results by PD-L1 expression.
Table 9. Efficacy results by PD-L1 expression in KEYNOTE-006:
| Endpoint | Pembrolizumab 10 mg/kg bw every 2 or 3 weeks (pooled) | Ipilimumab | Pembrolizumab 10 mg/kg bw every 2 or 3 weeks (pooled) | Ipilimumab |
|---|---|---|---|---|
| PD L1 positive | PD L1 negative | |||
| PFS Hazard ratio* (95% CI) | 0.53 (0.44, 0.65) | --- | 0.87 (0.58, 1.30) | --- |
| OS Hazard ratio* (95% CI) | 0.63 (0.50, 0.80) | --- | 0.76 (0.48, 1.19) | --- |
| ORR % | 40% | 14% | 24% | 13% |
* Hazard ratio (pembrolizumab compared to ipilimumab) based on the stratified Cox proportional hazard model
Ocular melanoma
In 20 subjects with ocular melanoma included in KEYNOTE-001, no objective responses were reported; stable disease was reported in 6 patients.
KEYNOTE-716: Placebo-controlled study for the adjuvant treatment of patients with resected Stage IIB or IIC melanoma
The efficacy of pembrolizumab was evaluated in KEYNOTE-716, a multicentre, randomised, double-blind, placebo-controlled study in patients with resected Stage IIB or IIC melanoma. A total of 976 patients were randomised (1:1) to receive pembrolizum ab 200 mg every three weeks (or the paediatric [12 to 17 years old] dose of 2 mg/kg intravenously [up to a maximum of 200 mg] every three weeks) (n=487) or placebo (n=489), for up to one year or until disease recurrence or unacceptable toxicity. Randomisation was stratified by American Joint Committee on Cancer (AJCC) 8th edition T stage. Patients with active autoimmune disease or a medical condition that required immunosuppression or mucosal or ocular melanoma were ineligible. Patients who received prior therapy for melanoma other than surgery were ineligible. Patients underwent imaging every six months from randomisation through the 4th year, and then once in year 5 from randomisation or until recurrence, whichever came first.
Among the 976 patients, the baseline characteristics were: median age of 61 years (range: 16-87; 39% age 65 or older; 2 adolescent patients [one per treatment arm]); 60% male; and ECOG PS of 0 (93%) and 1 (7%). Sixty-four percent had Stage IIB and 35% had Stage IIC.
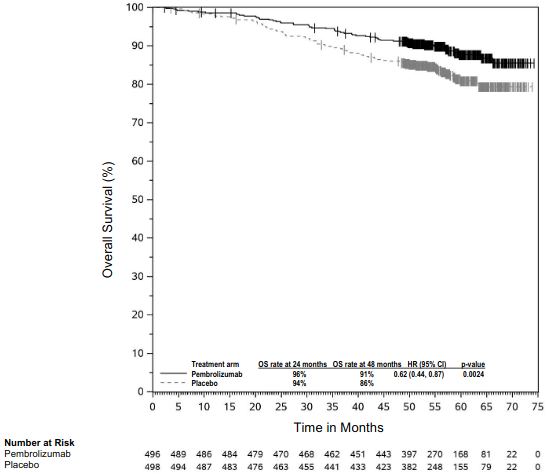
The primary efficacy outcome measure was investigator-assessed recurrence-free survival (RFS) in the whole population, where RFS was defined as the time between the date of randomisation and the date of first recurrence (local, regional, or distant metastasis) or death, whichever occurred first. The secondary outcome measures were distant metastasis-free survival (DMFS) and OS in the whole population. OS was not formally assessed at the time of this analysis. The study initially demonstrated a statistically significant improvement in RFS (HR 0.65; 95% CI 0.46, 0.92; p-value = 0.00658) for patients randomised to the pembrolizumab arm compared with placebo at its pre-specified interim analysis. Results reported from the pre-specified final analysis for RFS at a me dian follow-up of 20.5 months are summarised in Table 10. Updated RFS results at a median follow-up of 38.5 months were consistent with the final analysis for RFS for patients randomised to the pembrolizumab arm compared with placebo (HR 0.62; 95% CI 0.49, 0.79) (see Figure 4). The study demonstrated a statistically significant improvement in DMFS (HR 0.64; 95% CI 0.47, 0.88; p-Value = 0.00292) for patients randomised to the pembrolizumab arm compared with placebo at its pre-specified interim analysis at a median follow-up of 26.9 months. Results reported from the pre-specified final analysis for DMFS at a median follow-up time of 38.5 months are summarised in Table 10 and Figure 5.
Table 10. Efficacy results in KEYNOTE-716:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=487 | Placebo n=489 |
|---|---|---|
| RFS | ||
| Number (%) of patients with event | 72 (15%) | 115 (24%) |
| Median in months (95% CI) | NR (NR, NR) | NR (29.9, NR) |
| Hazard ratio* (95% CI) | 0.61 (0.45, 0.82) | |
| p-Value (stratified log-rank)† | 0.00046 | |
| DMFS | ||
| Number (%) of patients with event | 74 (15.2%) | 119 (24.3%) |
| Median in months (95% CI) | NR (NR, NR) | NR (NR, NR) |
| Hazard ratio* (95% CI) | 0.59 (0.44, 0.79) | |
* Based on the stratified Cox proportional hazard model
† Nominal p-Value based on log-rank test stratified by American Joint Committee on Cancer (AJCC) 8th edition T stage.
NR = not reached
Figure 4. Kaplan-Meier curve for recurrence-free survival by treatment arm in KEYNOTE-716 (intent to treat population):
Figure 5. Kaplan-Meier curve for distant metastasis-free survival by treatment arm in KEYNOTE-716 (intent to treat population):
KEYNOTE-054: Placebo-controlled study for the adjuvant treatment of patients with completely resected Stage III melanoma
The efficacy of pembrolizumab was evaluated in KEYNOTE-054, a multicentre, randomised, double-blind, placebo-controlled study in patients with completely resected stage IIIA (>1 mm lymph node metastasis), IIIB or IIIC melanoma. A total of 1 019 adult patients were randomised (1:1) to receive pembrolizumab 200 mg every three weeks (n=514) or placebo (n=505), for up to one year until disease recurrence or unacceptable toxicity. Randomisation was stratified by AJCC 7th edition stage (IIIA vs. IIIB vs. IIIC 1-3 positive lymph nodes vs. IIIC ≥4 positive lymph nodes) and geographic region (North America, European countries, Australia and other countries as designated). Patients must have undergone lymph node dissection, and if indicated, radiotherapy within 13 weeks prior to starting treatment. Patients with active autoimmune disease or a medical condition that required immunosuppression or mucosal or ocular melanoma were ineligible. Patients who received prior therapy for melanoma other than surgery or interferon for thick primary melanomas without evidence of lymph node involvement were ineligible. Patients underwent imaging every 12 weeks after the first dose of pembrolizumab for the first two years, then every 6 months from year 3 to 5, and then annually.
Among the 1 019 patients, the baseline characteristics were: median age of 54 years (25% age 65 or older); 62% male; and ECOG PS of 0 (94%) and 1 (6%). Sixteen percent had stage IIIA; 46% had stage IIIB; 18% had stage IIIC (1-3 positive lymph nodes) and 20% had stage IIIC (≥4 positive lymph nodes); 50% were BRAF V600 mutation positive and 44% were BRAF wild-type. PD-L1 expression was tested retrospectively by IHC assay with the 22C3 anti-PD-L1 antibody; 84% of patients had PD-L1-positive melanoma (PD-L1 expression in ≥1% of tumour and tumour-associated immune cells relative to all viable tumour cells). The same scoring system was used for metastatic melanoma (MEL score).
The primary efficacy outcome measures were investigator-assessed RFS in the whole population and in the population with PD-L1 positive tumours, where RFS was defined as the time between the date of randomisation and the date of first recurrence (local, regional, or distant metastasis) or death, whichever occurred first. The secondary outcome measures were DMFS and OS in the whole population and in the population with PD-L1 positive tumours. OS was not formally assessed at the time of these analyses. The study initially demonstrated a statistically significant improvement in RFS (HR 0.57; 98.4% CI 0.43, 0.74; p-value <0.0001) for patients randomised to the pembrolizumab arm compared with placebo at its pre-specified interim analysis. Updated efficacy results with a median follow-up time of 45.5 months are summarised in Table 11 and Figures 6 and 7.
Table 11. Efficacy results in KEYNOTE-054:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=514 | Placebo n=505 |
|---|---|---|
| RFS | ||
| Number (%) of patients with event | 203 (40%) | 288 (57%) |
| Median in months (95% CI) | NR | 21.4 (16.3, 27.0) |
| Hazard ratio* (95% CI) | 0.59 (0.49, 0.70) | |
| DMFS | ||
| Number (%) of patients with event | 173 (34%) | 245 (49%) |
| Median in months (95% CI) | NR | 40.0 (27.7, NR) |
| Hazard ratio* (95% CI) | 0.60 (0.49, 0.73) | |
| p-Value (stratified log-rank) | <0.0001 | |
* Based on the stratified Cox proportional hazard model
NR = not reached
Figure 6. Kaplan-Meier curve for recurrence-free survival by treatment arm in KEYNOTE-054 (intent to treat population):
Figure 7. Kaplan-Meier curve for distant metastasis-free survival by treatment arm in KEYNOTE-054 (intent to treat population):
RFS and DMFS benefit was consistently demonstrated across subgroups, including tumour PD-L1 expression, BRAF mutation status, and stage of disease (using AJCC 7th edition). These results were consistent when reclassified in a post-hoc analysis according to the current AJCC 8th edition staging system.
Non-small cell lung carcinoma
KEYNOTE-671: Controlled study for the neoadjuvant and adjuvant treatment of patients with resectable non-small cell lung carcinoma (NSCLC)
The efficacy of pembrolizumab in combination with platinum-containing chemotherapy, given as neoadjuvant treatment and continued as monotherapy as adjuvant treatment was investigated in KEYNOTE-671, a multicentre, randomised, double-blind, placebo-controlled study. Key eligibility criteria were previously untreated and resectable patients with NSCLC who are at high risk (Stage II, IIIA, or IIIB (N2) by AJCC 8th edition) of recurrence, regardless of tumour PD-L1 expression based on the PD-L1 IHC 22C3 pharmDxTM Kit. Testing for genomic tumour aberrations or oncogenic drivers was not mandatory for enrolment.
The following selection criteria define patients with high risk of recurrence who are included in the therapeutic indication and are reflective of the patient population with Stage II – IIIB (N2) according to the 8th edition staging system: tumour size >4 cm; or tumours of any size that are either accompanied by N1 or N2 status; or tumours that invade thoracic structures (directly invade the parietal pleura, chest wall, diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina); or tumours that involve a mainstem bronchus with tu mour >4 cm; or tumours >4 cm that cause obstructive atelectasis that extends to the hilum; or tumours with separate nodule(s) in the same lobe or different ipsilateral lobe as the primary lung cancer.
If indicated, patients received adjuvant radiation therapy prior to adjuvant pembrolizumab or placebo. Patients with active autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomisation was stratified by stage (II vs. III), tumour PD-L1 expression (TPS ≥50% or <50%), histology (squamous vs. non-squamous), and geographic region (East Asia vs. non-East Asia).
Patients were randomised (1:1) to one of the following treatment arms:
- Treatment Arm A: neoadjuvant pembrolizumab 200 mg on Day 1 in combination with cisplatin 75 mg/m² and either pemetrexed 500 mg/m² on Day 1 or gemcitabine 1 000 mg/m² on Days 1 and 8 of each 21-day cycle for up to 4 cycles. Following surgery, pembrolizumab 200 mg was administered every 3 weeks for up to 13 cycles.
- Treatment Arm B: neoadjuvant placebo on Day 1 in combination with cisplatin 75 mg/m² and either pemetrexed 500 mg/m² on Day 1 or gemcitabine 1 000 mg/m² on Days 1 and 8 of each 21-day cycle for up to 4 cycles. Following surgery, placebo was administered every 3 weeks for up to 13 cycles.
All study medications were administered via intravenous infusion. Treatment with pembrolizumab or placebo continued until completion of the treatment (17 cycles), disease progression that precluded definitive surgery, disease recurrence in the adjuvant phase, disease progression for those who did not undergo surgery or had incomplete resection and entered the adjuvant phase, or unacceptable toxicity. Assessment of tumour status was performed at baseline, Week 7, and Week 13 in the neoadjuvant phase, and within 4 weeks prior to the start of the adjuvant phase. Following the start of the adjuvant phase, assessment of tumour status was performed every 16 weeks through the end of Year 3, and then every 6 months thereafter.
The primary efficacy outcome measures were OS and investigator-assessed event-free survival (EFS). Secondary efficacy outcome measures were pathological complete response (pCR) rate and major pathological response (mPR) rate as assessed by blinded independent pathology review (BIPR).
A total of 797 patients in KEYNOTE-671 were randomised: 397 patients to the pembrolizumab arm and 400 to the placebo arm. Baseline characteristics were: median age of 64 years (range: 26 to 83), 45% age 65 or older; 71% male; 61% White, 31% Asian, and 2% Black. Sixty-three percent and 37% had ECOG performance of 0 or 1, respectively; 30% had Stage II and 70% had Stage III disease; 33% had TPS ≥50% and 67% had TPS <0%; 43% had tumours with squamous histology and 57% had tumours with non-squamous histology; 31% were from the East Asian region. Four percent of patients had EGFR mutations and in 66% EGFR mutation status was unknown. Three percent of patients had ALK translocations and in 68% ALK translocation status was unknown.
Eighty-one percent of patients in the pembrolizumab in combination with platinum-containing chemotherapy arm had definitive surgery compared to 76% of patients in the platinum-containing chemotherapy arm.
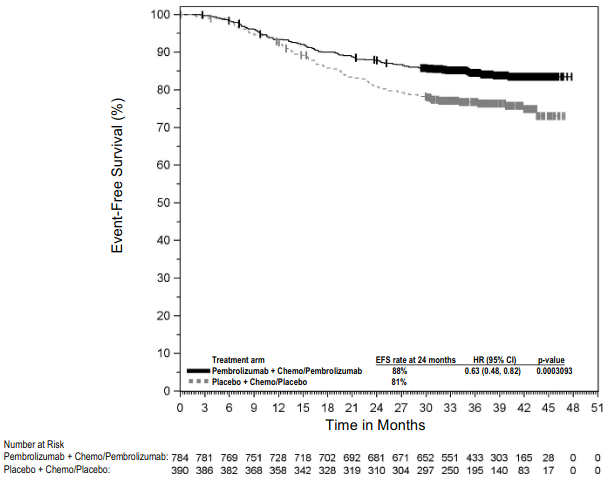
The study demonstrated statistically significant improvements in OS, EFS, pCR and mPR for patients randomised to pembrolizumab in combination with platinum-containing chemotherapy followed by pembrolizumab monotherapy compared with patients randomised to placebo in combination with platinum-containing chemotherapy followed by placebo alone. At a pre-specified interim analysis (median follow-up time of 21.4 months (range: 0.4 to 50.6 months)) the EFS HR was 0.58 (95% CI: 0.46, 0.72; p<0.0001) for patients randomised to pembrolizumab in combination with platinum-containing chemotherapy foll owed by pembrolizumab monotherapy compared with patients randomised to placebo in combination with platinum-containing chemotherapy followed by placebo alone. At the time of this analysis, OS results were not mature.
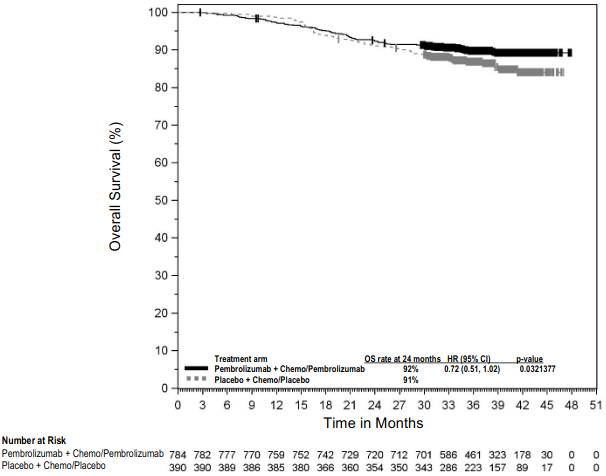
Table 12 summarises key efficacy measures at a pre-specified interim analysis at a median follow-up time of 29.8 months (range: 0.4 to 62.0 months). The Kaplan-Meier curves for OS and EFS are shown in Figures 8 and 9.
Table 12. Efficacy results in KEYNOTE-671:
| Endpoint | Pembrolizumab with chemotherapy/ Pembrolizumab n=397 | Placebo with chemotherapy/ Placebo n=400 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 110 (28%) | 144 (36%) |
| Median in months* (95% CI) | NR (NR, NR) | 52.4 (45.7, NR) |
| Hazard ratio† (95% CI) | 0.72 (0.56, 0.93) | |
| p-Value‡ | 0.00517 | |
| EFS | ||
| Number (%) of patients with event | 174 (44%) | 248 (62%) |
| Median in months* (95% CI) | 47.2 (32.9, NR) | 18.3 (14.8, 22.1) |
| Hazard ratio† (95% CI) | 0.59 (0.48, 0.72) | |
* Based on Kaplan-Meier estimates
† Based on Cox regression model with treatment as a covariate stratified by stage, tumour PD-L1 expression, histology, and geographic region
‡ Based on stratified log-rank test
NR = not reached
Figure 8. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-671 (intent to treat population):
Figure 9. Kaplan-Meier curve for event-free survival by treatment arm in KEYNOTE-671 (intent to treat population):
A post-hoc exploratory subgroup analysis was performed in KEYNOTE-671 in patients who had PD-L1 TPS ≥50% (pembrolizumab arm [n=132; 33%] vs. placebo arm [n=134; 34%]); TPS = 1-49% (pembrolizumab arm [n=127; 32%] vs. placebo arm [n=115; 29%]) and TPS <1% (pembrolizumab arm [n=138; 35%] vs. placebo arm [n=151; 38%]). The EFS HR was, 0.48 (95% CI: 0.33, 0.71) in patients with a TPS ≥50%, 0.52 (95% CI: 0.36, 0.73) in patients with a TPS = 1-49% and 0.75 (95% CI: 0.56, 1.01) in patients with a TPS <1%. The OS HR was 0.55 (95% CI: 0.33, 0.92) in patients with a TPS ≥50%, 0.69 (95% CI: 0.44, 1.07) in patients with a TPS = 1-49% and 0.91 (95% CI: 0.63, 1.32) in patients with a TPS <1%.
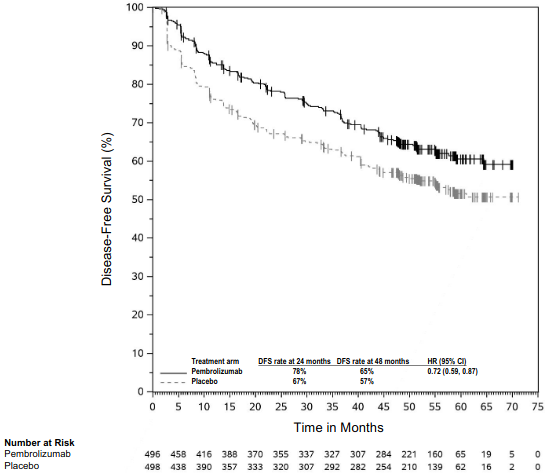
KEYNOTE-091: Placebo-controlled study for the adjuvant treatment of patients with resected NSCLC
The efficacy of pembrolizumab was investigated in KEYNOTE-091, a multicentre, randomised, triple-blind, placebo-controlled study in patients with NSCLC who are at high risk (stage IB [T2a ≥4 cm], II or IIIA by AJCC 7th edition) of recurrence following complete resection, regardless of tumour PD-L1 expression status, no prior neoadjuvant radiotherapy and/or neoadjuvant chemotherapy, and no prior or planned adjuvant radiotherapy for the current malignancy. Testing for genomic tumour aberrations/oncogenic drivers was not mandatory for enrolment.
The following selection criteria define patients with high risk of recurrence who are included in the therapeutic indication and are reflective of the patient population with stage IB [T2a ≥4 cm], II or IIIA according to the 7th edition staging system: Tumour size ≥4 cm; or tumours of any size that are either accompanied by N1 or N2 status; or tumours that are invasive of thoracic structures (directly invade the parietal pleura, chest wall, diaphragm, phrenic nerve, mediastinal pleura, parietal pericardiu m, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina); or tumours that involve the main bronchus <2 cm distal to the carina but without involvement of the carina; or tumours that are associated with atelectasis or obstructive pneumonitis of the entire lung; or tumours with separate nodule(s) in the same lobe or different ipsilateral lobe as the primary. The study did not include patients who had N2 status with tumours also invading the mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina, or with separate tumour nodule(s) in a different ipsilateral lobe.
Patients may or may not have received adjuvant chemotherapy as recommended by their physician. Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 4 cycles of adjuvant chemotherapy were ineligible. Randomisation was stratified by stage (IB vs. II vs. IIIA), adjuvant chemotherapy (no adjuvant chemotherapy vs. adjuvant chemotherapy), PD-L1 status (TPS <1% [negative] vs. TPS 1-49% vs. TPS ≥50%), and geographic region (Western Europe vs. Eastern Europe vs. Asia vs. Rest of World). Patients were randomised (1:1) to receive pembrolizumab 200 mg (n=590) or placebo (n=587) intravenously every 3 weeks.
Treatment continued until RECIST 1.1-defined disease recurrence as determined by the investigator, unacceptable toxicity, or approximately 1 year (18 doses). Patients underwent imaging every 12 weeks after the first dose of pembrolizumab for the first year, then every 6 months for years 2 to 3, and then annually up to the end of year 5. After year 5, imaging is performed as per local standard of care.
Of 1 177 patients randomised, 1 010 (86%) received adjuvant platinum-based chemotherapy following complete resection. Among these 1 010 patients in KEYNOTE-091, baseline characteristics were: median age of 64 years (range: 35 to 84), 49% age 65 or older; 68% male; and 77% White, 18% Asian, 86% current or former smokers. Sixty-one percent and 39% had ECOG performance of 0 or 1, respectively. Twelve percent had stage IB (T2a ≥4 cm), 57% had stage II, and 31% had stage IIIA disease. Thirty-nine percent had tumour PD-L1 expression TPS <1% [negative], 33% had TPS 1-49%, 28% had TPS ≥50%. Seven percent had known EGFR mutations, thirty-eight percent without EGFR mutations and in fifty-six percent EGFR mutation status was unknown. Fifty-two percent were from Western Europe, 20% from Eastern Europe, 17% from Asia, and 11% from Rest of World.
The primary efficacy outcome measures were investigator-assessed disease-free survival (DFS) in the overall population and in the population with tumour PD-L1 expression TPS ≥50% where DFS was defined as the time between the date of randomisation and the date of first recurrence (local/regional recurrence, distant metastasis), a second malignancy, or death, whichever occurred first. Secondary efficacy outcome measures were investigator-assessed DFS in the population with tumour PD-L1 expression TPS ≥1%, and OS in the overall population and in the populations with tumour PD-L1 expression TPS ≥50% and TPS ≥1%.
The study demonstrated a statistically significant improvement in DFS in the overall population (HR = 0.76 [95% CI: 0.63, 0.91; p = 0.0014]) at a pre-specified interim analysis with a median follow-up time of 32.4 months (range: 0.6 to 68 months) for patients randomised to the pembrolizumab arm compared to patients randomised to the placebo arm. Table 13 and Figure 10 summarise efficacy results in patients who received adjuvant chemotherapy at the final analysis for DFS performed at a median follow-up time of 46.7 months (range: 0.6 to 84.2). At the time of this analysis, OS results were not mature with only 58% of pre -specified OS events in the overall population. An exploratory analysis of OS suggested a trend in favour of pembrolizumab compared to placebo with a HR of 0.79 (95% CI: 0.62, 1.01) in patients who received adjuvant chemotherapy.
Table 13. Efficacy results in KEYNOTE-091 for patients who received adjuvant chemotherapy:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=506 | Placebo n=504 |
|---|---|---|
| DFS | ||
| Number (%) of patients with event | 225 (44%) | 262 (52%) |
| Hazard ratio* (95% CI) | 0.76 (0.64, 0.91) | |
| Median in months (95% CI) | 53.8 (46.2, 70.4) | 40.5 (32.9, 47.4) |
* Based on the multivariate Cox regression model.
Figure 10. Kaplan-Meier curve for disease-free survival by treatment arm in KEYNOTE-091 (for patients who received adjuvant chemotherapy):
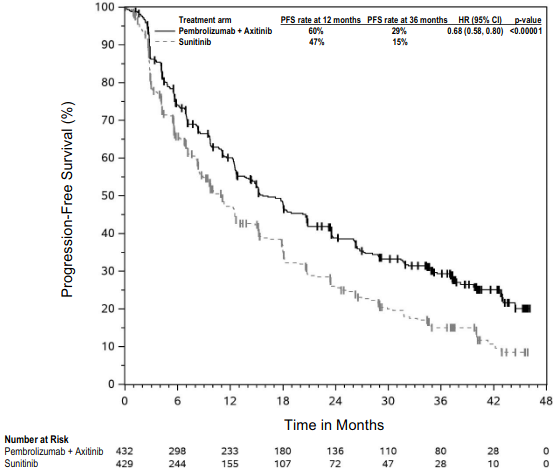
KEYNOTE-024: Controlled study of NSCLC patients naïve to treatment
The safety and efficacy of pembrolizumab were investigated in KEYNOTE-024, a multicentre, open-label, controlled study for the treatment of previously untreated metastatic NSCLC. Patients had PD-L1 expression with a ≥50% TPS based on the PD-L1 IHC 22C3 pharmDxTM Kit. Patients were randomised (1:1) to receive pembrolizumab at a dose of 200 mg every 3 weeks (n=154) or investigator's choice platinum-containing chemotherapy (n=151; including pemetrexed+carboplatin, pemetrexed+cisplatin, gemcitabine+cisplatin, gemcitabine+carboplatin, or paclitaxel+carbopl atin. Patients with non-squamous NSCLC could receive pemetrexed maintenance.). Patients were treated with pembrolizumab until unacceptable toxicity or disease progression. Treatment could continue beyond disease progression if the patient was clinically st able and was considered to be deriving clinical benefit by the investigator. Patients without disease progression could be treated for up to 24 months. The study excluded patients with EGFR or ALK genomic tumour aberrations; autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks. Assessment of tumour status was performed every 9 weeks. Patients on chemotherapy who experienced independently-verified progression of disease were able to crossover and receive pembrolizumab.
Among the 305 patients in KEYNOTE-024, baseline characteristics were: median age 65 years (54% age 65 or older); 61% male; 82% White, 15% Asian; and ECOG performance status 0 and 1 in 35% and 65%, respectively. Disease characteristics were squamous (18%) and non-squamous (82%); M1 (99%); and brain metastases (9%).
The primary efficacy outcome measure was PFS as assessed by blinded independent central review (BICR) using RECIST 1.1. Secondary efficacy outcome measures were OS and ORR (as assessed by BICR using RECIST 1.1). Table 14 summarises key efficacy measures for the entire intent to treat (ITT) population. PFS and ORR res ults are reported from an interim analysis at a median follow-up of 11 months. OS results are reported from the final analysis at a median follow-up of 25 months.
Table 14. Efficacy results in KEYNOTE-024:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=154 | Chemotherapy n=151 |
|---|---|---|
| PFS | ||
| Number (%) of patients with event | 73 (47%) | 116 (77%) |
| Hazard ratio* (95% CI) | 0.50 (0.37, 0.68) | |
| p-Value† | <0.001 | |
| Median in months (95% CI) | 10.3 (6.7, NA) | 6.0 (4.2, 6.2) |
| OS | ||
| Number (%) of patients with event | 73 (47%) | 96 (64 %) |
| Hazard ratio* (95% CI) | 0.63 (0.47, 0.86) | |
| p-Value† | 0.002 | |
| Median in months (95% CI) | 30.0 (18.3, NA) | 14.2 (9.8, 19.0) |
| Objective response rate | ||
| ORR % (95% CI) | 45% (37, 53) | 28% (21, 36) |
| Complete response % | 4% | 1% |
| Partial response % | 41% | 27% |
| Response duration‡ | ||
| Median in months (range) | Not reached (1.9+, 14.5+) | 6.3 (2.1+, 12.6+) |
| % with duration ≥6 months | 88%§ | 59%¶ |
* Hazard ratio (pembrolizumab compared to chemotherapy) based on the stratified Cox proportional hazard model
† Based on stratified Log rank test
‡ Based on patients with a best overall response as confirmed complete or partial response
§ Based on Kaplan-Meier estimates; includes 43 patients with responses of 6 months or longer
¶ Based on Kaplan-Meier estimates; includes 16 patients with responses of 6 months or longer
NA = not available
Figure 11. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-024 (intent to treat population):
Figure 12. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-024 (intent to treat population):
In a subgroup analysis, a reduced survival benefit of pembrolizumab compared to chemotherapy was observed in the small number of patients who were never-smokers; however, due to the small number of patients, no definitive conclusions can be drawn from these data.
KEYNOTE-042: Controlled study of NSCLC patients naïve to treatment
The safety and efficacy of pembrolizumab were also investigated in KEYN OTE-042, a multicentre, controlled study for the treatment of previously untreated locally advanced or metastatic NSCLC. The study design was similar to that of KEYNOTE-024, except that patients had PD-L1 expression with a ≥1% TPS based on the PD-L1 IHC 22C3 pharmDxTM Kit. Patients were randomised (1:1) to receive pembrolizumab at a dose of 200 mg every 3 weeks (n=637) or investigator's choice platinum-containing chemotherapy (n=637; including pemetrexed+carboplatin or paclitaxel+carboplatin. Patients with non-squamous NSCLC could receive pemetrexed maintenance). Assessment of tumour status was performed every 9 weeks for the first 45 weeks, and every 12 weeks thereafter.
Among the 1 274 patients in KEYNOTE-042, 599 (47%) had tumours that expressed PD-L1 with TPS ≥50% based on the PD-L1 IHC 22C3 pharmDxTM Kit. The baseline characteristics of these 599 patients included: median age 63 years (45% age 65 or older); 69% male; 63% White and 32% Asian; 17% Hispanic or Latino; and ECOG performance status 0 and 1 in 31% and 69%, respectively. Disease characteristics were squamous (37%) and non-squamous (63%); stage IIIA (0.8%); stage IIIB (9%); stage IV (90%); and treated brain metastases (6%).
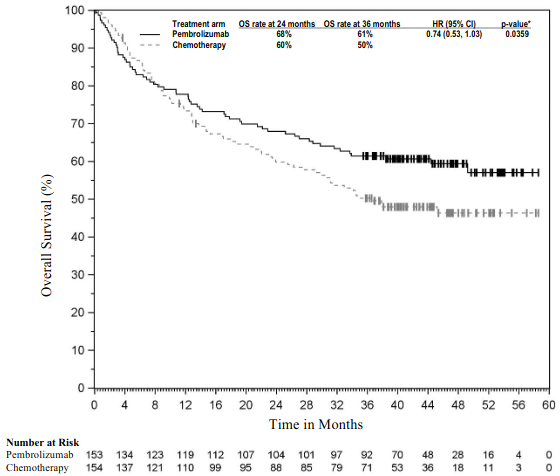
The primary efficacy outcome measure was OS. Secondary efficacy outcome measures were PFS and ORR (as assessed by BICR using RECIST 1.1). The study demonstrated a statistically significant improvement in OS for patients whose tumours expressed PD-L1 TPS ≥1% randomised to pembrolizumab monotherapy compared to chemotherapy (HR 0.82; 95% CI 0.71, 0.93 at the final analysis) and in patients whose tumours expressed PD-L1 TPS ≥50% randomised to pembrolizumab monotherapy compared to chemotherapy. Table 15 summarises key efficacy measures for the TPS ≥50% population at the final analysis performed at a median follow-up of 15.4 months. The Kaplan-Meier curve for OS for the TPS ≥50% population based on the final analysis is shown in Figure 13.
Table 15. Efficacy results (PD-L1 TPS ≥50%) in KEYNOTE-042:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=299 | Chemotherapy n=300 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 180 (60%) | 220 (73%) |
| Hazard ratio* (95% CI) | 0.70 (0.58, 0.86) | |
| p-Value† | 0.0003 | |
| Median in months (95% CI) | 20.0 (15.9, 24.2) | 12.2 (10.4, 14.6) |
| PFS | ||
| Number (%) of patients with event | 238 (80%) | 250 (83%) |
| Hazard ratio* (95% CI) | 0.84 (0.70, 1.01) | |
| Median in months (95% CI) | 6.5 (5.9, 8.5) | 6.4 (6.2, 7.2) |
| Objective response rate | ||
| ORR % (95% CI) | 39% (34, 45) | 32% (27, 38) |
| Complete response | 1% | 0.3% |
| Partial response | 38% | 32% |
| Response duration‡ | ||
| Median in months (range) | 22.0 (2.1+, 36.5+) | 10.8 (1.8+, 30.4+) |
| % with duration ≥18 months | 57% | 34% |
* Hazard ratio (pembrolizumab compared to chemotherapy) based on the stratified. Cox proportional hazard model.
† Based on stratified log-rank test.
‡ Based on patients with a best objective response as confirmed complete or partial response.
Figure 13. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-042 (patients with PD -L1 expression TPS ≥50%, intent to treat population):
The results of a post-hoc exploratory subgroup analysis indicated a trend towards reduced survival benefit of pembrolizumab compared to chemotherapy, during both the first 4 months and throughout the entire duration of treatment, in patients who were never-smokers. However, due to the exploratory nature of this subgroup analysis, no definitive conclusions can be drawn.
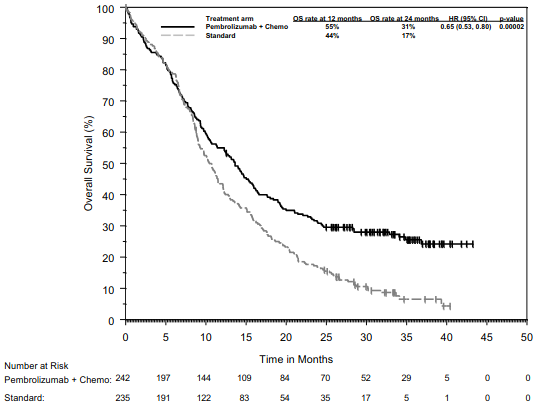
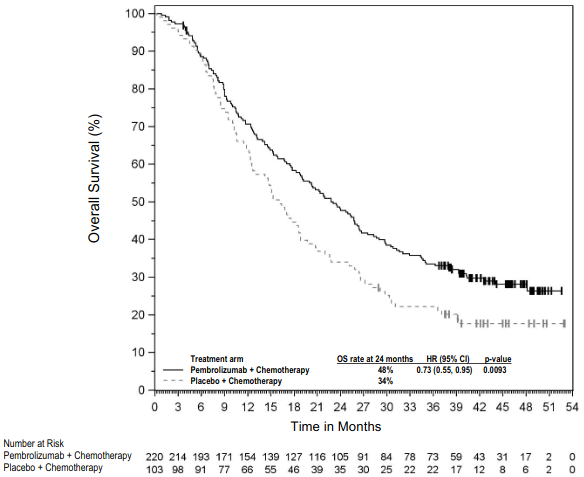
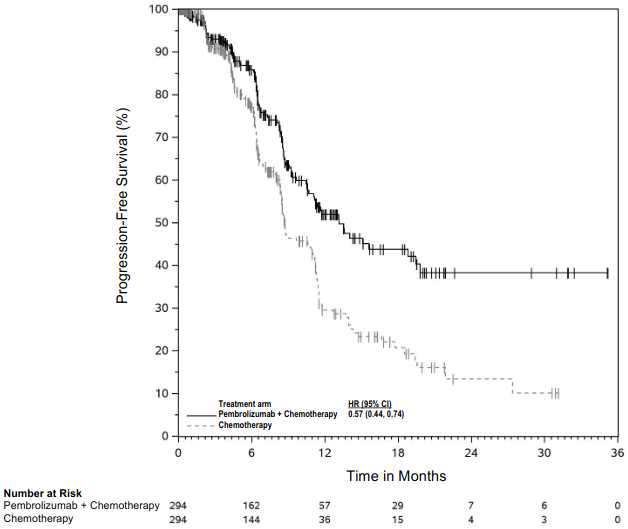
KEYNOTE-189: Controlled study of combination therapy in non-squamous NSCLC patients naïve to treatment
The efficacy of pembrolizumab in combination with pemetrexed and platinum chemotherapy was investigated in a multicentre, randomised, active-controlled, double-blind study, KEYNOTE-189. Key eligibility criteria were metastatic non-squamous NSCLC, no prior systemic treatment for metastatic NSCLC, and no EGFR or ALK genomic tumour aberrations. Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible. Patients were randomised (2:1) to receive one of the following regimens:
- Pembrolizumab 200 mg with pemetrexed 500 mg/m² and investigator's choice of cisplatin 75 mg/m² or carboplatin AUC 5 mg/mL/min intravenously every 3 weeks for 4 cycles followed by pembrolizumab 200 mg and pemetrexed 500 mg/m² intravenously every 3 weeks (n=410)
- Placebo with pemetrexed 500 mg/m² and investigator's choice of cisplatin 75 mg/m² or carboplatin AUC 5 mg/mL/min intravenously every 3 weeks for 4 cycles followed by placebo and pemetrexed 500 mg/m² intravenously every 3 weeks (n=206)
Treatment with pembrolizumab continued until RECIST 1.1-defined progression of disease as determined by the investigator, unacceptable toxicity, or a maximum of 24 months. Administration of pembrolizumab was permitted beyond RECIST-defined disease progression by BICR or beyond discontinuation of pemetrexed if the patient was clinically stable and deriving clinical benefit as determined by the investigator. For patients who completed 24 months of therapy or had a complete response, treatment with pembrolizumab could be reinitiated for disease progression and administered for up to 1 additional year. Assessment of tumo ur status was performed at Week 6 and Week 12, followed by every 9 weeks thereafter. Patients receiving placebo plus chemotherapy who experienced independently-verified progression of disease were offered pembrolizumab as monotherapy.
Among the 616 patients in KEYNOTE-189, baseline characteristics were: median age of 64 years (49% age 65 or older); 59% male; 94% White and 3% Asian; 43% and 56% ECOG performance status of 0 or 1 resp ectively; 31% PD -L1 negative (TPS <1%); and 18% with treated or untreated brain metastases at baseline.
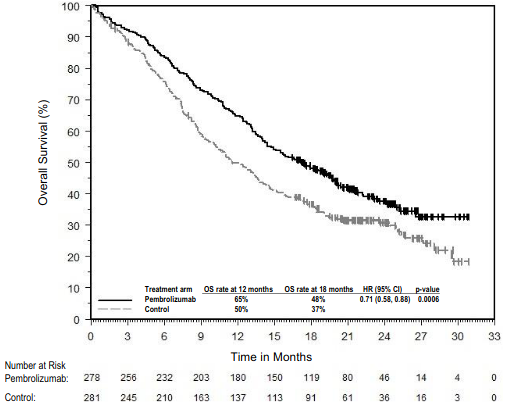
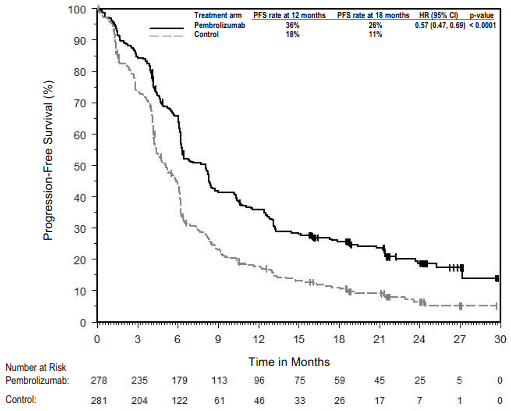
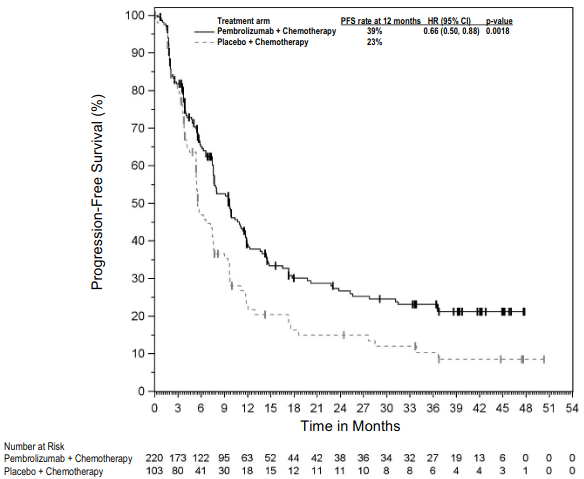
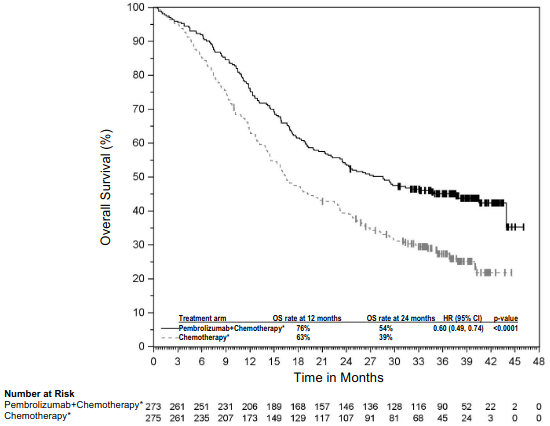
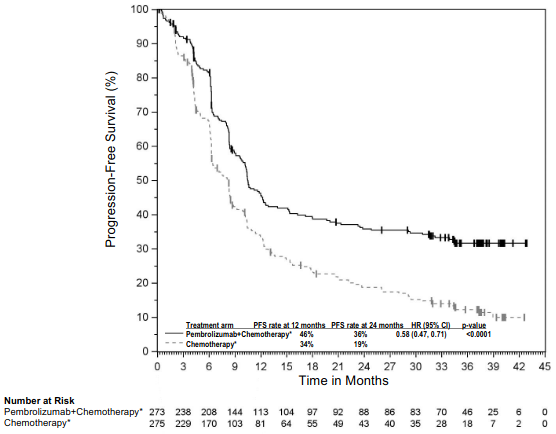
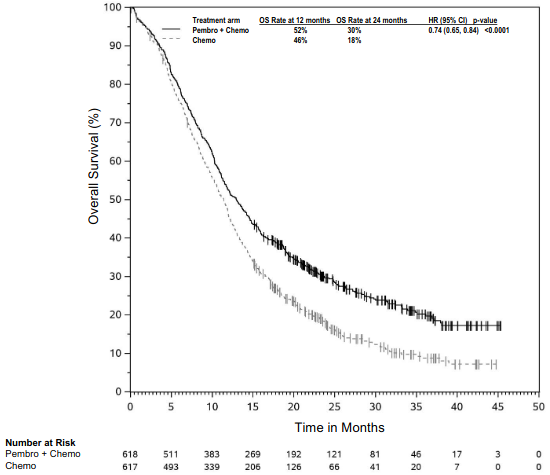
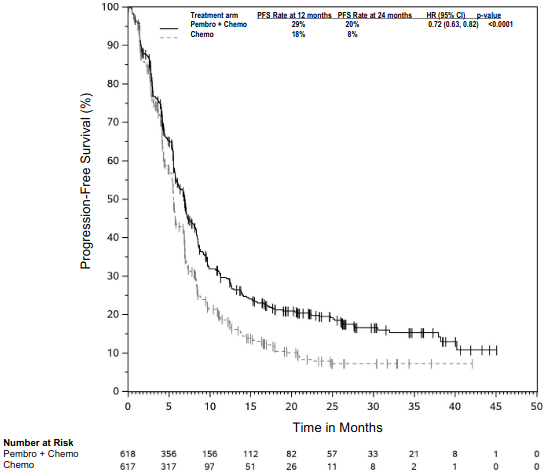
The primary efficacy outcome measures were OS and PFS (as assessed by BICR using RECIST 1.1). Secondary efficacy outcome measures were ORR and respons e duration, as assessed by BICR using RECIST 1.1. Table 16 summarises key efficacy measures and Figures 14 and 15 show the Kaplan-Meier curves for OS and PFS based on the final analysis with a median follow-up of 18.8 months.
Table 16. Efficacy results in KEYNOTE-189:
| Endpoint | Pembrolizumab + Pemetrexed + Platinum Chemotherapy n=410 | Placebo + Pemetrexed + Platinum Chemotherapy n=206 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 258 (63%) | 163 (79%) |
| Hazard ratio† (95% CI) | 0.56 (0.46, 0.69) | |
| p-Value‡ | <0.00001 | |
| Median in months (95% CI) | 22.0 (19.5, 24.5) | 10.6 (8.7, 13.6) |
| PFS | ||
| Number (%) of patients with event | 337 (82%) | 197 (96%) |
| Hazard ratio† (95% CI) | 0.49 (0.41, 0.59) | |
| p-Value‡ | <0.00001 | |
| Median in months (95% CI) | 9.0 (8.1, 10.4) | 4.9 (4.7, 5.5) |
| Objective response rate | ||
| ORR§ % (95% CI) | 48% (43, 53) | 20% (15, 26) |
| Complete response | 1.2% | 0.5% |
| Partial response | 47% | 19% |
| p-Value¶ | <0.0001 | |
| Response duration | ||
| Median in months (range) | 12.5 (1.1+, 34.9+) | 7.1 (2.4, 27.8+) |
| % with duration ≥12 months# | 53% | 27% |
* A total of 113 patients (57%) who discontinued study treatment in the placebo plus chemotherapy arm crossed over to receive monotherapy pembrolizumab or received a checkpoint inhibitor as subsequent therapy
† Based on the stratified Cox proportional hazard model
‡ Based on stratified log-rank test
§ Based on patients with a best objective response as confirmed complete or partial response
¶ Based on Miettinen and Nurminen method stratified by PD - L1 status, platinum chemotherapy and smoking status
# Based on Kaplan-Meier estimation
Figure 14: Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-189 (intent to treat population):
Figure 15. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-189 (intent to treat population):
An analysis was performed in KEYNOTE-189 in patients who had PD-L1 TPS <1% [pembrolizumab combination: n=127 (31%) vs. chemotherapy: n=63 (31%)], TPS 1-49% [pembrolizumab combination: n=128 (31%) vs. chemotherapy: n=58 (28%)] or ≥50% [pembrolizumab combination: n=132 (32%) vs. chemotherapy: n=70 (34%)] (see Table 17).
Table 17. Efficacy results by PD-L1 expression in KEYNOTE-189*:
| Endpoint | Pembrolizumab combination therapy | Chemotherapy | Pembrolizumab combination therapy | Chemotherapy | Pembrolizumab combination therapy | Chemotherapy |
|---|---|---|---|---|---|---|
| TPS <1% | TPS 1 to 49% | TPS ≥50% | ||||
| OS Hazard ratio† (95% CI) | 0.51 (0.36, 0.71) | 0.66 (0.46, 0.96) | 0.59 (0.40, 0.86) | |||
| PFS Hazard ratio† (95% CI) | 0.67 (0.49, 0.93) | 0.53 (0.38, 0.74) | 0.35 (0.25, 0.49) | |||
| ORR % | 33% | 14% | 50% | 21% | 62% | 26% |
* Based on final analysis
† Hazard ratio (pembrolizumab combination therapy compared to chemotherapy) based on the stratified Cox proportional hazard model
At final analysis, a total of 57 NSCLC patients aged ≥75 years were enrolled in study KEYNOTE-189 (35 in the pembrolizumab combination and 22 in the control). A HR=1.54 [95% CI 0.76, 3.14] in OS and HR=1.12 [95% CI 0.56, 2.22] in PFS for pembrolizumab combination vs. chemotherapy was reported within this study subgroup. Data about efficacy of pembrolizumab in combination with platinum chemotherapy are limited in this patient population.
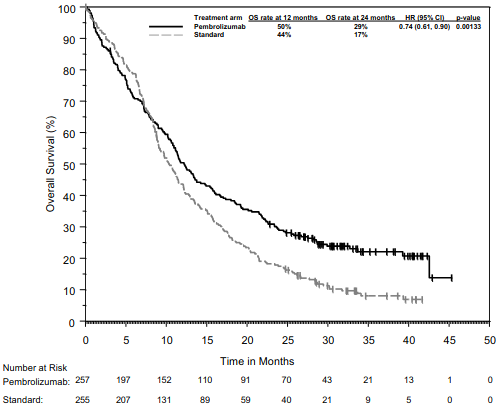
KEYNOTE-407: Controlled study of combination therapy in squamous NSCLC patients naïve to treatment
The efficacy of pembrolizumab in combination with carboplatin and either paclitaxel or nab-paclitaxel was investigated in Study KEYNOTE-407, a randomised, double-blind, multicentre, placebo-controlled study. The key eligibility criteria for this study were metastatic squamous NSCLC, regardless of tumour PD-L1 expression status, and no prior systemic treatment for metastatic disease. Patients with autoimmune disease that required systemic therapy within 2 years of treatment; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks were ineligible. Randomisation was stratified by tumour PD-L1 expression (TPS <1% [negative] vs. TPS ≥1%), investigator's choice of paclitaxel or nab-paclitaxel, and geographic region (East Asia vs. non-East Asia). Patients were randomised (1:1) to one of the following treatment arms via intravenous infusion:
- Pembrolizumab 200 mg and carboplatin AUC 6 mg/mL/min on Day 1 of each 21-day cycle for 4 cycles, and paclitaxel 200 mg/m² on Day 1 of each 21-day cycle for 4 cycles or nab-paclitaxel 100 mg/m² on Days 1, 8 and 15 of each 21-day cycle for 4 cycles, followed by pembrolizumab 200 mg every 3 weeks. Pembrolizumab was administered prior to chemotherapy on Day 1.
- Placebo and carboplatin AUC 6 mg/mL/min on Day 1 of each 21 -day cycle for 4 cycles and paclitaxel 200 mg/m² on Day 1 of each 21-day cycle for 4 cycles or nab-paclitaxel 100 mg/m² on Days 1, 8 and 15 of each 21 -day cycle for 4 cycles, followed by placebo every 3 weeks.
Treatment with pembrolizumab or placebo continued until RECIST 1.1-defined progression of disease as determined by BICR, unacceptable toxicity, or a maximum of 24 months. Administration of pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and deriving clinical benefit as de termined by the investigator.
Patients in the placebo arm were offered pembrolizumab as a single agent at the time of disease progression.
Assessment of tumour status was performed every 6 weeks through Week 18, every 9 weeks through Week 45 and every 1 2 weeks thereafter.
A total of 559 patients were randomised. The study population characteristics were: median age of 65 years (range: 29 to 88); 55% age 65 or older; 81% male; 77% White; ECOG performance status of 0 (29%) and 1 (71%); and 8% with treate d brain metastases at baseline. Thirty-five percent had tumour PD-L1 expression TPS <1% [negative]; 19% were East Asian; and 60% received paclitaxel.
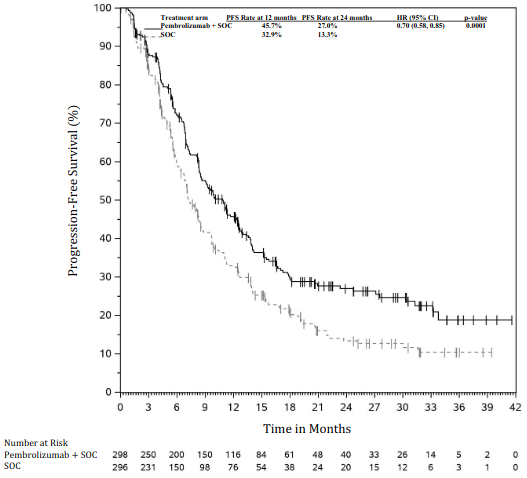
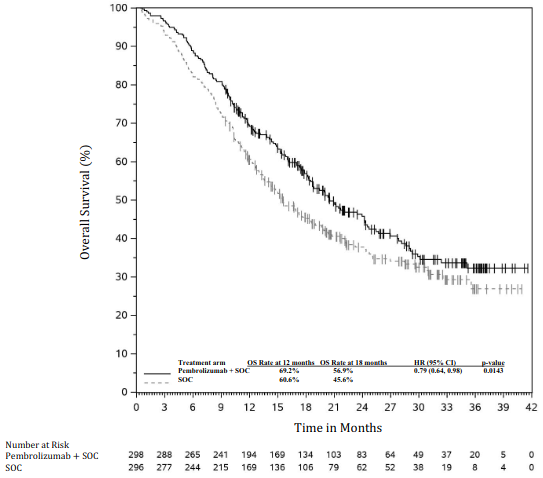
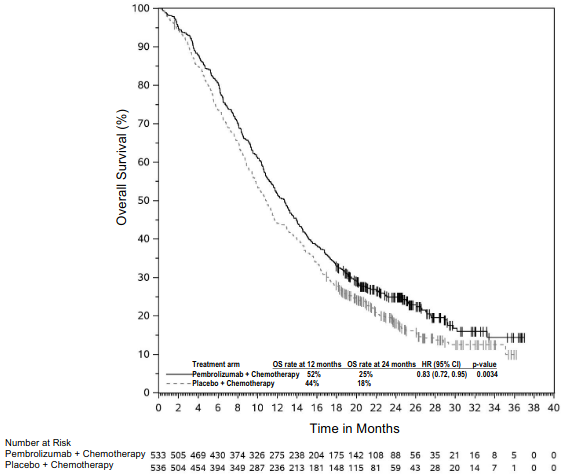
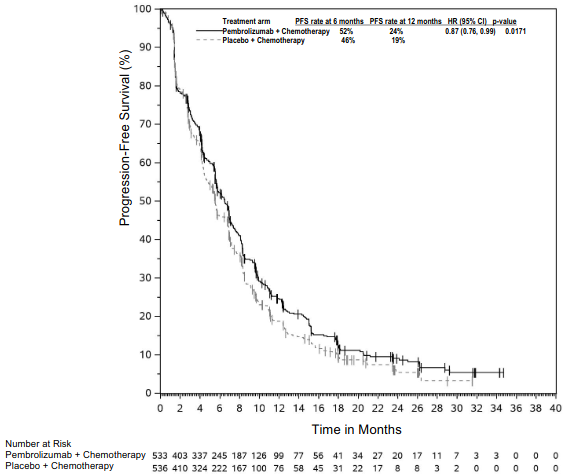
The primary efficacy outcome measures were OS and PFS (as assessed by BICR using RECIST 1.1). Secondary efficacy outcome measures were ORR and response duration, as assessed by BICR using RECIST 1.1. Table 18 summarises key efficacy measures and Figures 16 and 17 show the Kaplan-Meier curves for OS and PFS based on the final analysis with a median follow-up of 14.3 months.
Table 18. Efficacy results in KEYNOTE-407:
| Endpoint | Pembrolizumab Carboplatin Paclitaxel/Nab-paclitaxel n=278 | Placebo Carboplatin Paclitaxel/Nab-paclitaxel n=281 |
|---|---|---|
| OS* | ||
| Number (%) of patients with event | 168 (60%) | 197 (70%) |
| Median in months (95% CI) | 17.1 (14.4, 19.9) | 11.6 (10.1, 13.7) |
| Hazard ratio† (95% CI) | 0.71 (0.58, 0.88) | |
| p-Value‡ | 0.0006 | |
| PFS | ||
| Number (%) of patients with event | 217 (78%) | 252 (90%) |
| Median in months (95% CI) | 8.0 (6.3, 8.4) | 5.1 (4.3, 6.0) |
| Hazard ratio† (95% CI) | 0.57 (0.47, 0.69) | |
| p-Value‡ | <0.0001 | |
| Objective response rate | ||
| ORR % (95% CI) | 63% (57, 68) | 38% (33, 44) |
| Complete response | 2.2% | 3.2% |
| Partial response | 60% | 35% |
| p-Value§ | <0.0001 | |
| Response duration | ||
| Median in months (range) | 8.8 (1.3+, 28.4+) | 4.9 (1.3+, 28.3+) |
| % with duration ≥12 months¶ | 38% | 25% |
* A total of 138 patients (51%) who discontinued study treatment in the placebo plus chemotherapy arm crossed over to receive monotherapy pembrolizumab or received a checkpoint inhibitor as subsequent therapy
† Based on the stratified Cox proportional hazard model
‡ Based on stratified log-rank test
§ Based on method by Miettinen and Nurminen
¶ Based on Kaplan-Meier estimation
Figure 16. Kaplan-Meier Curve for Overall Survival in KEYNOTE-407:
Figure 17. Kaplan-Meier Curve for Progression-Free Survival in KEYNOTE-407:
An analysis was performed in KEYNOTE-407 in patients who had PD-L1 TPS <1% [pembrolizumab plus chemotherapy arm: n=95 (34%) vs. placebo plus chemotherapy arm: n=99 (35%)], TPS 1% to 49% [pembrolizumab plus chemo therapy arm: n=103 (37%) vs. placebo plus chemotherapy arm: n=104 (37%)] or TPS ≥50% [pembrolizumab plus chemotherapy arm: n=73 (26%) vs. placebo plus chemotherapy arm: n=73 (26%)] (see Table 19).
Table 19. Efficacy results by PD-L1 expression in KEYNOTE-407*:
| Endpoint | Pembrolizumab combination therapy | Chemotherapy | Pembrolizumab combination therapy | Chemotherapy | Pembrolizumab combination therapy | Chemotherapy |
|---|---|---|---|---|---|---|
| TPS <1% | TPS 1 to 49% | TPS ≥50% | ||||
| OS Hazard ratio† (95% CI) | 0.79 (0.56, 1.11) | 0.59 (0.42, 0.84) | 0.79 (0.52, 1.21) | |||
| PFS Hazard ratio† (95% CI) | 0.67 (0.49, 0.91) | 0.52 (0.38, 0.71) | 0.43 (0.29, 0.63) | |||
| ORR % | 67% | 41% | 55% | 42% | 64% | 30% |
* Based on final analysis
† Hazard ratio (pembrolizumab combination therapy compared to chemotherapy) based on the stratified Cox proportional hazard model
At final analysis, a total of 65 NSCLC patients aged ≥75 years were enrolled in study KEYNOTE-407 (34 in the pembrolizumab combination and 31 in the control). An HR=0.81 [95% CI 0.43, 1.55] in OS, an HR=0.61 [95% CI 0.34, 1.09] in PFS, and an ORR of 62% and 45% for pembrolizumab combination vs. chemotherapy was reported within this study subgroup. Data about efficacy of pembrolizumab in combination with platinum chemotherapy are limited in this patient population.
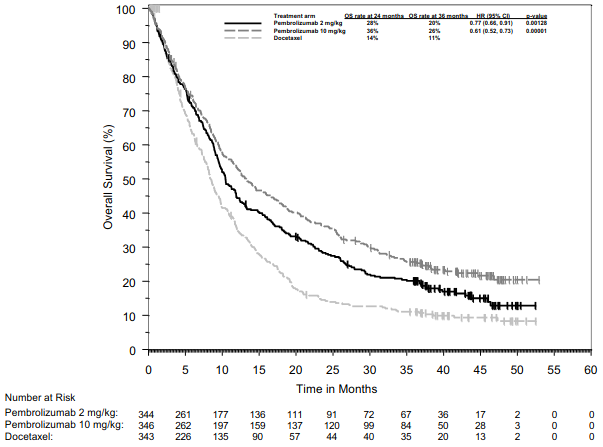
KEYNOTE-010: Controlled study of NSCLC patients previously treated with chemotherapy
The safety and efficacy of pembrolizumab were investigated in KEYNOTE-010, a multicentre, open-label, controlled study for the treatment of advanced NSCLC in patients previously treated with platinum-containing chemotherapy. Patients had PD-L1 expression with a ≥1% TPS based on the PD-L1 IHC 22C3 pharmDxTM Kit. Patients with EGFR activation mutatio n or ALK translocation also had disease progression on approved therapy for these mutations prior to receiving pembrolizumab. Patients were randomised (1:1:1) to receive pembrolizumab at a dose of 2 (n=344) or 10 mg/kg bw (n=346) every 3 weeks or docetaxel at a dose of 75 mg/m² every 3 weeks (n=343) until disease progression or unacceptable toxicity. The study excluded patients with autoimmune disease; a medical condition that required immunosuppression; or who had received more than 30 Gy of thoracic radiation within the prior 26 weeks. Assessment of tumour status was performed every 9 weeks.
The baseline characteristics for this population included: median age 63 years (42% age 65 or older); 61% male; 72% White and 21% Asian and 34% and 66% with an ECOG performance status 0 and 1, respectively. Disease characteristics were squamous (21%) and non-squamous (70%); stage IIIA (2%); stage IIIB (7%); stage IV (91%); stable brain metastases (15%) and the incidence of mutations was EGFR (8%) or ALK (1%). Prior therapy included platinum-doublet regimen (100%); patients received one (69%) or two or more (29%) treatment lines.
The primary efficacy outcome measures were OS and PFS as assessed by BICR using RECIST 1.1. Secondary efficacy outcome measures were ORR and response duration. Table 20 summarises key efficacy measures for the entire population (TPS ≥1%) and for the patients with TPS ≥50%, and Figure 18 shows the Kaplan-Meier curve for OS (TPS ≥1%), based on a final analysis with median follow-up of 42.6 months.
Table 20. Response to pembrolizumab 2 or 10 mg/kg bw every 3 weeks in previously treated patients with NSCLC in KEYNOTE-010:
| Endpoint | Pembrolizumab 2 mg/kg bw every 3 weeks | Pembrolizumab 10 mg/kg bw every 3 weeks | Docetaxel 75 mg/m² every 3 weeks |
|---|---|---|---|
| TPS ≥1% | |||
| Number of patients | 344 | 346 | 343 |
| OS | |||
| Number (%) of patients with event | 284 (83%) | 264 (76%) | 295 (86%) |
| Hazard ratio* (95% CI) | 0.77 (0.66, 0.91) | 0.61 (0.52, 0.73) | --- |
| p-Value† | 0.00128 | <0.001 | --- |
| Median in months (95% CI) | 10.4 (9.5, 11.9) | 13.2 (11.2, 16.7) | 8.4 (7.6, 9.5) |
| PFS‡ | |||
| Number (%) of patients with event | 305 (89%) | 292 (84%) | 314 (92%) |
| Hazard ratio* (95% CI) | 0.88 (0.75, 1.04) | 0.75 (0.63, 0.89) | --- |
| p-Value† | 0.065 | <0.001 | --- |
| Median in months (95% CI) | 3.9 (3.1, 4.1) | 4.0 (2.7, 4.5) | 4.1 (3.8, 4.5) |
| Objective response rate‡ | |||
| ORR % (95% CI) | 20% (16, 25) | 21% (17, 26) | 9% (6, 13) |
| Complete response | 2% | 3% | 0% |
| Partial response | 18% | 18% | 9% |
| Response duration‡,§ | |||
| Median in months (range) | Not reached (2.8, 46.2+) | 37.8 (2.0+, 49.3+) | 7.1 (1.4+, 16.8) |
| % ongoing¶ | 42% | 43% | 6% |
| TPS ≥50% | |||
| Number of patients | 139 | 151 | 152 |
| OS | |||
| Number (%) of patients with event | 97 (70%) | 102 (68%) | 127 (84%) |
| Hazard ratio* (95% CI) | 0.56 (0.43, 0.74) | 0.50 (0.38, 0.65) | --- |
| p-Value† | <0.001 | <0.001 | --- |
| Median in months (95% CI) | 15.8 (10.8, 22.5) | 18.7 (12.1, 25.3) | 8.2 (6.4, 9.8) |
| PFS‡ | |||
| Number (%) of patients with event | 107 (77%) | 115 (76%) | 138 (91%) |
| Hazard ratio* (95% CI) | 0.59 (0.45, 0.77) | 0.53 (0.41, 0.70) | --- |
| p-Value† | <0.001 | <0.001 | --- |
| Median in months (95% CI) | 5.3 (4.1, 7.9) | 5.2 (4.1, 8.1) | 4.2 (3.8, 4.7) |
| Objective response rate‡ | |||
| ORR % (95% CI) | 32% (24, 40) | 32% (25, 41) | 9% (5, 14) |
| Complete response | 4% | 4% | 0% |
| Partial response | 27% | 28% | 9% |
| Response duration‡,§ | |||
| Median in months (range) | Not reached (2.8, 44.0+) | 37.5 (2.0+, 49.3+) | 8.1 (2.6, 16.8) |
| % ongoing¶ | 55% | 47% | 8% |
* Hazard ratio (pembrolizumab compared to docetaxel) based on the stratified Cox proportional hazard model
† Based on stratified log-rank test
‡ Assessed by BICR using RECIST 1.1
§ Based on patients with a best objective response as confirmed complete or partial response
¶ Ongoing response includes all responders who at the time of analysis were alive, progression-free, did not initiate new anti-cancer therapies and had not been determined to be lost to follow-up
Figure 18. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-010 (patients with PD-L1 expression TPS ≥1%, intent to treat population):
Efficacy results were similar for the 2 mg/kg bw and 10 mg/kg bw pembrolizumab arms. Efficacy results for OS were consistent regardless of the age of tumour specimen (new vs. archival) based on an intergroup comparison.
In subgroup analyses, a reduced survival benefit of pembrolizumab compared to docetaxel was observed for patients who were never-smokers or patients with tumours harbouring EGFR activating mutations who received at least platinum-based chemotherapy and a tyrosine kinase inhibitor; however, due to the small numbers of patients, no definitive conclusions can be drawn from these data.
The efficacy and safety of pembrolizumab in patients with tumours that do not express PD-L1 have not been established.
Malignant pleural mesothelioma
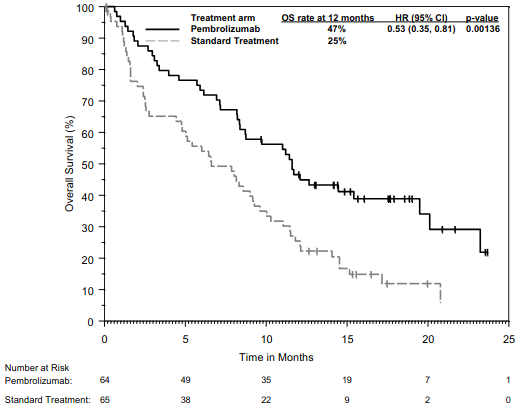
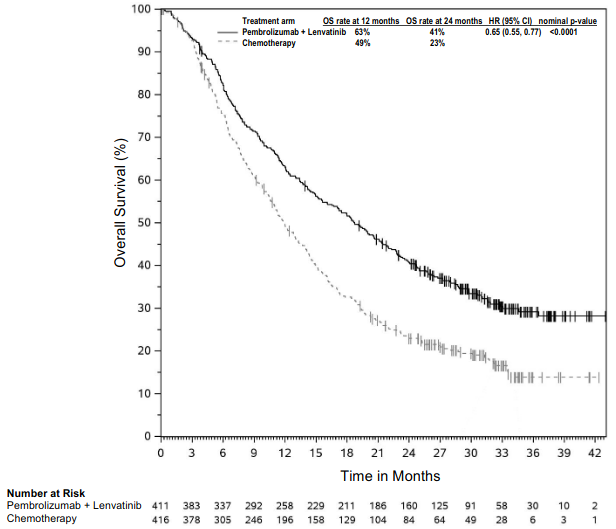
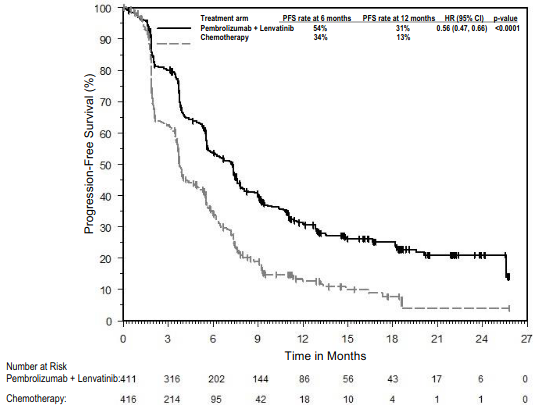
KEYNOTE-483: Controlled study of combination therapy in patients with untreated unresectable advanced or metastatic malignant pleural mesothelioma (MPM)
The efficacy of pembrolizumab in combination with pemetrexed and platinum chemotherapy was investigated in KEYNOTE-483, a multicentre, randomised, open-label, active-controlled study. Key eligibility criteria were unresectable advanced or metastatic MPM with no prior systemic therapy for advanced/metastatic disease. Patients were enrolled regardless of tumour PD-L1 expression. Patients with autoimmune disease that required systemic therapy within 3 years of treatment or a medical condition that required immunosuppression were ineligible. Randomisation was stratified by histological subtype (epithelioid vs. non-epithelioid). Patients were randomised (1:1) to one of the following treatment arms; all study medications were administered via intravenous infusion:
- Pembrolizumab 200 mg with pemetrexed 500 mg/m² and cisplatin 75 mg/m² or carboplatin AUC 5-6 mg/mL/min on Day 1 of each 21-day cycle for up to 6 cycles, ollowed by pembrolizumab 200 mg every 3 weeks (n=222). Pembrolizumab was administered prior to chemotherapy on Day 1.
- Pemetrexed 500 mg/m² and cisplatin 75 mg/m² or carboplatin AUC 5-6 mg/mL/min on Day 1 of each 21-day cycle for up to 6 cycles (n=218).
Treatment with pembrolizumab continued until disease progression as determined by the investigator according to modified RECIST 1.1 for mesothelioma (mRECIST), unacceptable toxicity, or a maximum of 24 months. Assessment of tumour status was performed every 6 weeks for 18 weeks, followed by every 12 weeks thereafter.
Among the 95 patients with non-epithelioid histology in KEYNOTE -483, baseline characteristics were: median age of 71 years (range: 48-85 years of age) with 76% age 65 or older; 83% male; 85% White, 15% not reported or unknown; 1% Hispanic or Latino and 44% and 56% ECOG performance status of 0 or 1, respectively.
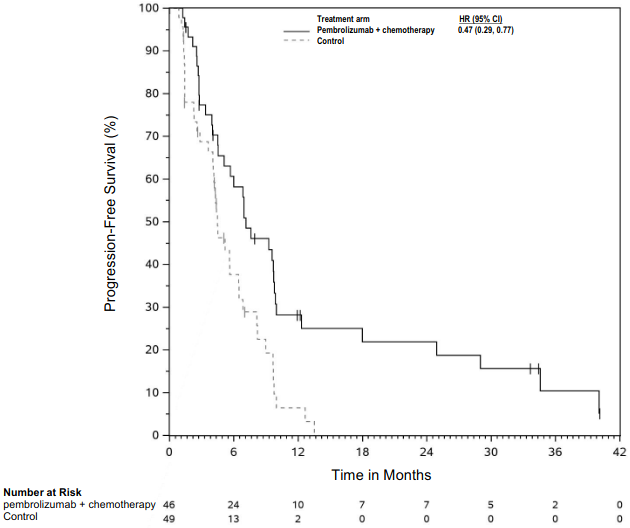
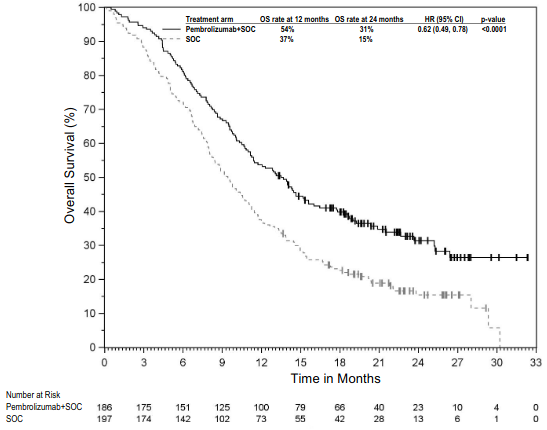
The primary efficacy outcome measure was OS. Additional efficacy outcome measures were PFS, ORR, and DoR, as assessed by BICR using mRECIST. The study demonstrated statistically significant improvement in the overall population in OS [0.79 (95% CI 0.64, 0.98; p-Value 0.0162)] and PFS [0.80 (95% CI 0.65, 0.99; p-Value 0.0194)] at the final analysis and ORR [52% (95% CI 45, 59)] vs. [29% (95% CI 23, 3 5) p-Value <0.00001] at the interim analysis in patients randomised to pembrolizumab in combination with chemotherapy compared with patients randomised to chemotherapy alone. Table 21 summarises key efficacy measures and Figures 19 and 20 show the Kaplan-Meier curves for OS and PFS based on the final analysis with a median follow-up time of 9.8 months (range: 0.9 to 60.3 months) in the patients with non-epithelioid malignant pleural mesothelioma.
Table 21. Efficacy results in KEYNOTE-483 for patients with non-epithelioid malignant pleural mesothelioma:
| Endpoint | Pembrolizumab 200 mg every 3 weeks + Pemetrexed + Platinum Chemotherapy (n=46) | Pemetrexed + Platinum Chemotherapy (n=49) |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 37 (80%) | 44 (90%) |
| Hazard ratio* (95% CI) | 0.57 (0.36, 0.89) | |
| Median in months† (95% CI) | 12.3 (8.7, 21.2) | 8.2 (5.8, 9.8) |
| PFS | ||
| Number (%) of patients with event | 36 (78%) | 38 (78%) |
Hazard ratio* (95% CI) |\2<>0.47 (0.29, 0.77) |
|Median in months† (95% CI) |<>7.1 (4.5, 9.8) |<>4.5 (4.0, 6.4) |
|\3< Objective response rate |
|ORR % (95% CI)‡ |<>41% (27, 57) |<>6% (1, 17) |
|\3< Response duration† |
|Median in months (range) |<>11.1 (1.3+, 38.9+) |<>4.0 (2.4+, 5.2) |
* Based on Cox regression model with Efron's method of tie handling with treatment as a covariate
† From product-limit (Kaplan-Meier) method for censored data
‡ Based on the exact method for binomial data
Figure 19. Kaplan-Meier curve for overall survival in patients with non-epithelioid MPM in KEYNOTE-483:
Figure 20. Kaplan-Meier curve for progression-free survival in patients with non-epithelioid MPM in KEYNOTE-483:
Classical Hodgkin lymphoma
KEYNOTE-204: Controlled study in patients with relapsed or refractory classical Hodgkin lymphoma (cHL)
The efficacy of pembrolizumab was investigated in KEYNOTE-204, a randomised, open-label, active-controlled study conducted in 304 patients with relapsed or refractory cHL. Patients with active, non-infectious pneumonitis, an allogeneic HSCT within the past 5 years (or >5 years but with symptoms of GVHD), active autoimmune disease, a medical condition that required immunosuppression, or an active infection requiring systemic therapy were ineligible for the study. Randomisation was stratified by prior ASCT (yes vs. no) and disease status after frontline therapy (primary refractory vs. relapse less than 12 months after completion vs. relapse 12 months or more after completion). Patients were randomised (1:1) to one of the following treatment arms:
- Pembrolizumab 200 mg intravenously every 3 weeks
- Brentuximab vedotin (BV) 1.8 mg/kg bw intravenously every 3 weeks.
Patients received pembrolizumab 200 mg intravenously every 3 weeks until unacceptable toxicity or documented disease progression, or a maximum of 35 cycles. Limited data are currently available on response duration following pembrolizumab discontinuation at cycle 35. Response was assessed every 12 weeks, with the first planned post-baseline assessment at Week 12.
Among the 304 patients in KEYNOTE-204, there is a subpopulation consisting of 112 patients who failed a transplant before enrolling and 137 who failed 2 or more prior therapies and were ineligible for ASCT at the time of enrolment. The baseline characteristics of these 249 patients were: median age 34 years (11% age 65 or older); 56% male; 80% White and 7% Asian and 58% and 41% with an ECOG performance status 0 and 1, respectively. Approximately 30% were refractory to frontline chemotherapy and ~45% had received prior ASCT. Nodular-sclerosis was the more represented cHL histological subtype (~81%) and bulky disease, B symptoms and bone marrow involvement were present in approximately 21%, 28% and 4% of patients, respectively.
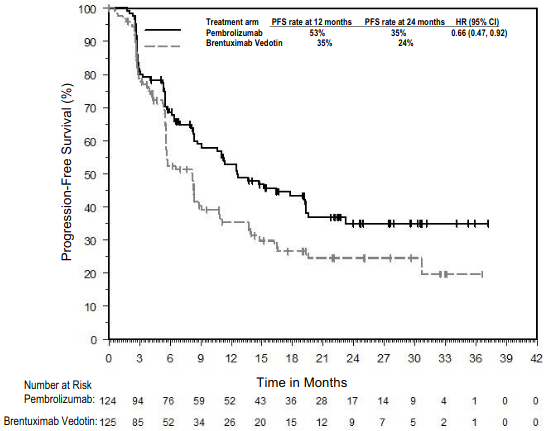
The primary efficacy outcome was PFS and the secondary efficacy outcome measure was ORR, both assessed by BICR according to the 2007 revised International Working Group (IWG) criteria. The additional primary efficacy outcome measure, OS, was not formally assessed at the time of the analysis. In the ITT population, the median follow-up time for 151 patients treated with pembrolizumab was 24.9 months (range: 1.8 to 42.0 months). The initial analysis resulted in a HR for PFS of 0.65 (95% CI: 0.48, 0.88) with a one-sided p value of 0.0027. The ORR was 66% for pembrolizumab compared to 54% for standard treatment with a p-Value of 0.0225. Table 22 summarises the efficacy results in the subpopulation. Efficacy results in this subpopulation were consistent with the ITT population. The Kaplan-Meier curve for PFS for this subpopulation is shown in Figure 21.
Table 22. Efficacy results in cHL patients who failed a transplant before enrolling or who failed 2 or more prior therapies and were ineligible for ASCT in KEYNOTE-204:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=124 | Brentuximab vedotin 1.8 mg/kg bw every 3 weeks n=125 |
|---|---|---|
| PFS | ||
| Number (%) of patients with event | 68 (55%) | 75 (60%) |
| Hazard ratio* (95% CI) | 0.66 (0.47, 0.92) | |
| Median in months (95% CI) | 12.6 (8.7, 19.4) | 8.2 (5.6, 8.8) |
| Objective response rate | ||
| ORR‡ % (95% CI) | 65% (56.3, 73.6) | 54% (45.3, 63.3) |
| Complete response | 27% | 22% |
| Partial response | 39% | 33% |
| Stable disease | 12% | 23% |
| Response duration | ||
| Median in months (range) | 20.5 (0.0+, 33.2+) | 11.2 (0.0+, 33.9+) |
| Number (%¶) of patients with duration ≥6 months | 53 (80.8%) | 28 (61.2%) |
| Number (%¶) of patients with duration ≥12 months | 37 (61.7%) | 17 (49.0%) |
* Based on the stratified Cox proportional hazard model
‡ Based on patients with a best overall response as complete or partial response
¶ Based on Kaplan-Meier estimation
Figure 21. Kaplan-Meier curve for progression-free survival by treatment arm in cHL patients who failed a transplant before enrolling or who failed 2 or more prior therapies and were ineligible for ASCT in KEYNOTE-204:
KEYNOTE-087 and KEYNOTE-013: Open-label studies in patients with relapsed or refractory cHL
The efficacy of pembrolizumab was investigated in KEYNOTE-087 and KEYNOTE-013, two multicentre, open-label studies for the treatment of 241 patients with cHL. These studies enrolled patients who failed ASCT and BV, who were ineligible for ASCT because they were unable to achieve a complete or partial remission to salvage chemotherapy and failed BV, or who failed ASCT and did not receive BV. Five study subjects were ineligible to ASCT due to reasons other than failure to salvage chemotherapy. Both studies included patients regardless of PD-L1 expression. Patients with active, non-infectious pneumonitis, an allogeneic transplant within the past 5 years (or >5 years but with GVHD), active autoimmune disease or a medical condition that required immunosuppression were ineligible for either study. Patients received pembrolizumab 200 mg every 3 weeks (n=210; KEYNOTE-087) or 10 mg/kg bw every 2 weeks (n=31; KEYNOTE-013) until unacceptable toxicity or documented disease progression.
Among KEYNOTE-087 patients, the baseline characteristics were median age 35 years (9% age 65 or older); 54% male; 88% White; and 49% and 51% had an ECOG performance status 0 and 1, respectively. The median number of prior lines of therapy administered for the treatment of cHL was 4 (range: 1 to 12). Eighty-one percent were refractory to at least one prior therapy, including 34% who were refractory to first-line therapy. Sixty-one percent of patients had received ASCT, 38% were transplant ineligible; 17% had no prior brentuximab vedotin use; and 37% of patients had prior radiation therapy. Disease subtypes were 81% nodular sclerosis, 11% mixed cellularity, 4% lymphocyte-rich and 2% lymphocyte-depleted.
Among KEYNOTE-013 patients, the baseline characteristics were median age 32 years (7% age 65 or older), 58% male, 94% White; and 45% and 55% had an ECOG performance status 0 and 1, respectively. The median number of prior lines of therapy administered for the treatment of cHL was 5 (range: 2 to 15). Eighty-four percent were refractory to at least one prior therapy, including 35% who were refractory to first-line therapy. Seventy-four percent of patients had re ceived ASCT, 26% were transplant ineligible, and 45% of patients had prior radiation therapy. Disease subtypes were 97% nodular sclerosis and 3% mixed cellularity.
The primary efficacy outcome measures (ORR and CRR) were assessed by BICR according to the IWG 2007 criteria. Secondary efficacy outcome measures were duration of response, PFS and OS. Response was assessed in KEYNOTE-087 and KEYNOTE-013 every 12 and 8 weeks, respectively, with the first planned post-baseline assessment at Week 12. Main efficacy results are summarised in Table 23.
Table 23. Efficacy results in KEYNOTE-087 and KEYNOTE-013:
| KEYNOTE-087* | KEYNOTE-013† | |
|---|---|---|
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=210 | Pembrolizumab 10 mg/kg bw every 2 weeks n=31 |
| Objective response rate‡ | ||
| ORR % (95% CI) | 71% (64.8, 77.4) | 58% (39.1, 75.5) |
| Complete remission | 28% | 19% |
| Partial remission | 44% | 39% |
| Response duration‡ | ||
| Median in months (range) | 16.6 (0.0+, 62.1+)§ | Not reached (0.0+, 45.6+)¶ |
| % with duration ≥12-months | 59%# | 70%Þ |
| % with duration ≥24-months | 45%ß | --- |
| % with duration ≥60-months | 25%à | --- |
| Time to response | ||
| Median in months (range) | 2.8 (2.1, 16.5)§ | 2.8 (2.4, 8.6)¶ |
| OS | ||
| Number (%) of patients with event | 59 (28%) | 6 (19%) |
| 12-month OS rate | 96% | 87% |
| 24-month OS rate | 91% | 87% |
| 60-month OS rate | 71% | --- |
* Median follow-up time of 62.9 months
† Median follow-up time of 52.8 months
‡ Assessed by BICR according to the IWG 2007 criteria by PET CT scans
§ Based on patients (n=150) with a response by independent review
¶ Based on patients (n=18) with a response by independent review
# Based on Kaplan-Meier estimation; includes 62 patients with responses of 12 months or longer
Þ Based on Kaplan-Meier estimation; includes 7 patients with responses of 12 months or longer
ß Based on Kaplan-Meier estimation; includes 37 patients with responses of 24 months or longer
à Based on Kaplan-Meier estimation; includes 4 patients with responses of 60 months or longer
Efficacy in elderly patients:
Overall, 46 cHL patients ≥65 years were treated with pembrolizumab in studies KEYNOTE-087, KEYNOTE-013 and KEYNOTE-204. Data from these patients are too limited to draw any conclusion on efficacy in this population.
Urothelial carcinoma
KEYNOTE-A39: Controlled study of combination therapy with enfortumab vedotin for the first-line treatment of unresectable or metastatic urothelial carcinoma
The efficacy of pembrolizumab in combination with enfortumab vedotin was investigated in KEYNOTE-A39, an open-label, multicentre, randomi sed, active-controlled study, that enrolled 886 patients with unresectable or metastatic urothelial carcinoma. The study excluded patients with autoimmune disease or a medical condition that required immunosuppression, active CNS metastases, ongoing sensory or motor neuropathy ≥ Grade 2, or uncontrolled diabetes defined as haemoglobin A1C (HbA1c) ≥8% or HbA1c ≥7% with associated diabetes symptoms, pneumonitis, or other forms of interstitial lung disease. Patients who received neoadjuvant chemotherapy or patients who received adjuvant chemotherapy following cystectomy were included in the study if recurrence was >12 months from completion of therapy. Patients were considered cisplatin-ineligible if they had at least one of the following criteria: glomerular filtration rate 30-59 mL/min, ECOG PS ≥2, Grade ≥2 hearing loss, or NYHA Class III heart failure. Patients randomised to the gemcitabine and platinum-based chemotherapy arm were permitted to receive maintenance immunotherapy.
Randomisation was stratified by cisplatin eligibility (eligible or ineligible), PD-L1 expression (CPS ≥10 or CPS <10 based on the PD -L1 IHC 22C3 pharmDxTM Kit), and liver metastases (present or absent). Patients were randomised (1:1) to one of the following treatment arms; all study medicati ons were administered via intravenous infusion;
- Pembrolizumab 200 mg over 30 minutes on Day 1 and enfortumab vedotin 1.25 mg/kg on Days 1 and 8 of each 21-day cycle.
- Gemcitabine 1 000 mg/m² on Days 1 and 8 and investigator's choice of cisplatin 70 mg/m² or carboplatin (AUC 4.5 or 5 mg/mL/min according to local guidelines) on Day 1 of each 21-day cycle.
Treatment with pembrolizumab and enfortumab vedotin continued until RECIST v1.1-defined progression of disease, unacceptable toxicity, or for pembrolizumab a maximum of 35 cycles (up to approximately 2 years). Assessment of tumour status was performed every 9 weeks for 18 months, and then every 12 weeks thereafter.
Among 886 patients with urothelial carcinoma, baseline characteristics were: median age of 69 years; 77% male; and 6 7% White. Ninety-five percent had M1 disease, and 5% had M0 disease. Seventy-three percent had a primary tumo ur in the lower tract, and 27% in the up per tract. Fifty-four percent was cisplatin-eligible, 58% had PD-L1 CPS ≥10, and 72% had visceral metastases, including 22% with liver metastases. Twenty percent had normal renal function, and 37%, 41% and 2% were characterised with mild, moderate, or severe renal impairment, respectively. Ninety-seven percent had ECOG PS of 0-1 and 3% had ECOG PS of 2. Eighty-five percent had transitional cell carcinoma (TCC) histology, 2% had TCC with other histology and 6% had TCC with squamous differentiation. Thirty-two percent of patients in the gemcitabine and platinum-based chemotherapy arm received maintenance immunotherapy.
The primary efficacy outcome measures were PFS as assessed by BICR according to RECIST v1.1 and OS. Secondary outcome measures were ORR and DoR as assessed by BICR according to RECIST v1.1 and time to pain progression (TTPP).
The study demonstrated a statistically significant improvement in OS, PFS and ORR in patients randomised to pembrolizumab in combination with enfortumab vedotin compared with patients randomised to gemcitabine and platinum-based chemotherapy.
The median follow-up time for 442 patients treated with pembrolizumab and enfortumab vedotin was 17.3 months (range: 0.3 to 37.2 months). Key efficacy results are summarised in Table 24 and Figures 22 and 23.
Table 24. Efficacy results in KEYNOTE-A39:
| Endpoint | Pembrolizumab 200 mg every 3 weeks in combination with Enfortumab vedotin n=442 | Gemcitabine + Platinum chemotherapy with or without maintenance immunotherapy n=444 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 133 (30%) | 226 (51%) |
| Median in months (95% CI) | 31.5 (25.4, NR) | 16.1 (13.9, 18.3) |
| Hazard ratio* (95% CI) | 0.47 (0.38, 0.58) | |
| p-Value† | <0.00001 | |
| PFS | ||
| Number (%) of patients with event | 223 (50%) | 307 (69%) |
| Median in months (95% CI) | 12.5 (10.4, 16.6) | 6.3 (6.2, 6.5) |
| Hazard ratio* (95% CI) | 0.45 (0.38, 0.54) | |
| p-Value† | <0.00001 | |
| Objective response rate‡ | ||
| ORR§ % (95% CI) | 68% (63.1, 72.1) | 44% (39.7, 49.2) |
| p-Value¶ | <0.00001 | |
| Response duration | ||
| Median in months (range) | NR (2.0+, 28.3+) | 7.0 (1.5+, 30.9+) |
* Based on the stratified Cox proportional hazard regression model
† Two-sided p-Value based on stratified log-rank test
‡ Includes only patients with measurable disease at baseline
§ Based on patients with a best overall response as confirmed complete or partial response
¶ Two-sided p-Value based on Cochran-Mantel-Haenszel test stratified by PD-L1 expression, cisplatin eligibility and liver metastases
NR = not reached
Figure 22. Kaplan-Meier curve for overall survival in KEYNOTE-A39:
Figure 23. Kaplan-Meier curve for progression-free survival in KEYNOTE-A39:
KEYNOTE-045: Controlled study in urothelial carcinoma patients who have received prior platinum-containing chemotherapy
The safety and efficacy of pembrolizumab were evaluated in KEYNOTE-045, a multicentre, open-label, randomised (1:1), controlled study for the treatment of locally advanced or metastatic urothelial carcinoma in patients with disease progression on or after platinum-containing chemotherapy. Patients must have received first-line platinum-containing regimen for locally advanced/metastatic disease or as neoadjuvant/adjuvant treatment, with recurrence/progression ≤12 months following completion of therapy. Patients were randomised (1:1) to receive either pembrolizumab 200 mg every 3 weeks (n=270) or investigator's choice of any of the following chemotherapy regimens all given intravenously every 3 weeks (n=272): paclitaxel 175 mg/m² (n=84), docetaxel 75 mg/m² (n=84), or vinflunine 320 mg/m² (n=87). Patients were treated with pembrolizumab until unacceptable toxicity or disease progression. Treatment could continue beyond progression if the patient was clinically stable and was considered to be deriving clinical benefit by the investigator. Patients without disease progression could be treated for up to 24 months. The study excluded patients with autoimmune disease, a medical condition that required immunosuppression and patients with more than 2 prior lines of systemic chemotherapy for metastatic urothelial carcinoma. Patients with an ECOG performance status of 2 had to have a haemoglobin ≥10 g/dL, could not have liver metastases, and must have received the last dose of their last prior chemotherapy regimen ≥3 months prior to enrolment. Assessment of tumour status was performed at 9 weeks after the first dose, then every 6 weeks through the first year, followed by every 12 weeks thereafter.
Among the 542 randomised patients in KEYNOTE-045, baseline characteristics were: median age 66 years (range: 26 to 88), 58% age 65 or older; 74% male; 72% White and 23% Asian; 56% ECOG performance status of 1 and 1% ECOG performance status of 2; and 96% M1 disease and 4% M0 disease. Eighty-seven percent of patients had visceral metastases, including 34% with liver metastases. Eighty-six percent had a primary tumour in the lower tract and 14% had a primary tumour in the upper tract. Fifteen percent of patients had disease progression following prior platinum-containing neoadjuvant or adjuvant chemotherapy. Twenty-one percent had received 2 prior systemic regimens in the metastatic setting. Seventy-six percent of patients received prior cisplatin, 23% had prior carboplatin, and 1% was treated with other platinum-based regimens.
The primary efficacy outcomes were OS and PFS as assessed by BI CR using RECIST v1.1. Secondary outcome measures were ORR (as assessed by BICR using RECIST v1.1) and duration of response. Table 25 summarises the key efficacy measures for the ITT population at the final analysis. The Kaplan-Meier curve based on the final analysis for OS is shown in Figure 24. The study demonstrated statistically significant improvements in OS and ORR for patients randomised to pembrolizumab as compared to chemotherapy. There was no statistically significant difference between pembrolizumab and chemotherapy with respect to PFS.
Table 25. Response to pembrolizumab 200 mg every 3 weeks in patients with urothelial carcinoma previously treated with chemotherapy in KEYNOTE-045:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=270 | Chemotherapy n=272 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 200 (74%) | 219 (81%) |
| Hazard ratio* (95% CI) | 0.70 (0.57, 0.85) | |
| p-Value† | <0.001 | |
| Median in months (95% CI) | 10.1 (8.0, 12.3) | 7.3 (6.1, 8.1) |
| PFS‡ | ||
| Number (%) of patients with event | 233 (86%) | 237 (87%) |
| Hazard ratio* (95% CI) | 0.96 (0.79, 1.16) | |
| p-Value† | 0.313 | |
| Median in months (95% CI) | 2.1 (2.0, 2.2) | 3.3 (2.4, 3.6) |
| Objective response rate‡ | ||
| ORR % (95% CI) | 21% (16, 27) | 11% (8, 15) |
| p-Value§ | <0.001 | |
| Complete response | 9% | 3% |
| Partial response | 12% | 8% |
| Stable disease | 17% | 34% |
| Response duration‡,¶ | ||
| Median in months (range) | Not reached (1.6+, 30.0+) | 4.4 (1.4+, 29.9+) |
| Number (%#) of patients with duration ≥6 months | 46 (84%) | 8 (47%) |
| Number (%#) of patients with duration ≥12 months | 35 (68%) | 5 (35%) |
* Hazard ratio (pembrolizumab compared to chemotherapy) based on the stratified Cox proportional hazard model
† Based on stratified log-rank test
‡ Assessed by BICR using RECIST 1.1
§ Based on method by Miettinen and Nurminen
¶ Based on patients with a best objective response as confirmed complete or partial response
# Based on Kaplan-Meier estimation
Figure 24. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-045 (intent to treat population):
An analysis was performed in KEYNOTE-045 in patients who had PD-L1 CPS <10 [pembrolizumab: n=186 (69%) vs. chemotherapy: n=176 (65%)] or ≥10 [pembrolizumab: n=74 (27%) vs. chemotherapy: n=90 (33%)] in both pembrolizumab - and chemotherapy-treated arms (see Table 25).
Table 26. OS by PD-L1 expression:
| PD-L1 Expression | Pembrolizumab | Chemotherapy | |
|---|---|---|---|
| OS by PD-L1 Expression Number (%) of patients with event* | Hazard Ratio† (95% CI) | ||
| CPS <10 | 140 (75%) | 144 (82%) | 0.75 (0.59, 0.95) |
| CPS ≥10 | 53 (72%) | 72 (80%) | 0.55 (0.37, 0.81) |
* Based on final analysis
† Hazard ratio (pembrolizumab compared to chemotherapy) based on the stratified Cox proportional hazard model
Patient-reported outcomes (PROs) were assessed using EORTC QLQ-C30. A prolonged time to deterioration in EORTC QLQ-C30 global health status/QoL was observed for patients treated with pembrolizumab compared to investigator's choice chemotherapy (HR 0.70; 95% CI 0.55-0.90). Over 15 weeks of follow-up, patients treated with pembrolizumab had stable global health status/QoL, while those treated with investigator's choice chemotherapy had a decline in global health status/QoL. These results should be interpreted in the context of the open-label study design and therefore taken cautiously.
KEYNOTE-052: Open-label study in urothelial carcinoma patients ineligible for cisplatin-containing chemotherapy
The safety and efficacy of pembrolizumab were investigated in KEYNOTE-052, a multicentre, open-label study for the treatment of locally advanced or metastatic urothelial carcinoma in patients who were not eligible for cisplatin-containing chemotherapy. Patients received pembrolizumab at a dose of 200 mg every 3 weeks until unacceptable toxicity or disease progression. Treatment could continue beyond progression if the patient was clinically stable and was considered to be deriving clinical benefit by the investigator. Patients without disease progression could be treated for up to 24 months. The study excluded patients with autoimmune disease or a medical condition that required immunosuppression. Assessment of tumour status was performed at 9 weeks after the first dose, then every 6 weeks through the first year, followed by every 12 weeks thereafter.
Among 370 patients with urothelial carcinoma who were not eligible for cisplatin-containing chemotherapy baseline characteristics were: median age 74 years (82% age 65 or older); 77% male; and 89% White and 7% Asian. Eighty-eight percent had M1 disease and 12% had M0 disease. Eighty-five percent of patients had visceral metastases, including 21% with liver metastases. Reasons for cisplatin ineligibility included: baseline creatinine clearance of <60 mL/min (50%), ECOG performance status of 2 (32%), ECOG performance status of 2 and baseline creatinine clearance of <60 mL/min (9%), and other (Class III heart failure, Grade 2 or greater peripheral neuropathy, and Grade 2 or greater hearing loss; 9%). Ninety percent of patients were treatment naïve, and 10% received prior adjuvant or neoadjuv ant platinum-based chemotherapy. Eighty-one percent had a primary tumour in the lower tract, and 19% of patients had a primary tumour in the upper tract.
The primary efficacy outcome measure was ORR as assessed by BICR using RECIST 1.1. Secondary efficacy outcome measures were duration of response, PFS, and OS. Table 27 summarises the key efficacy measures for the study population at the final analysis based on a median follow-up time of 11.4 months (range: 0.1, 41.2 months) for all patients.
Table 27. Response to pembrolizumab 200 mg every 3 weeks in patients with urothelial carcinoma ineligible for cisplatin-containing chemotherapy in KEYNOTE-052:
| Endpoint | n=370 |
|---|---|
| Objective response rate* | |
| ORR % (95% CI) | 29% (24, 34) |
| Disease control rate† | 47% |
| Complete response | 9% |
| Partial response | 20% |
| Stable disease | 18% |
| Response duration | |
| Median in months (range) | 30.1 (1.4+, 35.9+) |
| % with duration ≥6-months | 81%‡ |
| Time to response | |
| Median in months (range) | 2.1 (1.3, 9.0) |
| PFS* | |
| Median in months (95% CI) | 2.2 (2.1, 3.4) |
| 6-month PFS rate | 33% |
| 12-month PFS rate | 22% |
| OS | |
| Median in months (95% CI) | 11.3 (9.7, 13.1) |
| 6-month OS rate | 67% |
| 12-month OS rate | 47% |
* Assessed by BICR using RECIST 1.1
† Based on best response of stable disease or better
‡ Based on Kaplan-Meier estimates; includes 84 patients with response of 6 months or longer
An analysis was performed in KEYNOTE-052 in patients who had tumours that expressed PD-L1 with a CPS <10 (n=251; 68%) or ≥10 (n=110; 30%) based on the PD-L1 IHC 22C3 pharmDxTM Kit (see Table 28).
Table 28. ORR and OS by PD-L1 expression:
| Endpoint | CPS <10 n=251 | CPS ≥10 n=110 |
|---|---|---|
| Objective response rate* | ||
| ORR % (95% CI) | 20% (16, 26) | 47% (38, 57) |
| OS | ||
| Median in months (95% CI) | 10 (8, 12) | 19 (12, 29) |
| 12-month OS rate | 41% | 61% |
* BICR using RECIST 1.1
KEYNOTE-361 is a Phase III, randomised, controlled, open-label clinical study of pembrolizumab with or without platinum-based combination chemotherapy (i.e. either cisplatin or carboplatin with gemcitabine) versus chemotherapy as first-line treatment in subjects with advanced or metastatic urothelial carcinoma. Results of KEYNOTE-361 for pembrolizumab in combination with chemotherapy did not show statistically significant improvement in PFS as assessed by BICR using RECIST 1.1 (HR 0.78; 95% CI: 0.65, 0.93; p=0.0033), and OS (HR 0.86; 95% CI: 0.72, 1.02; p=0.0407) versus chemotherapy alo ne. Per the pre -specified hierarchical testing order no formal tests for statistical significance of pembrolizumab versus chemotherapy could be performed. The key efficacy results of pembrolizumab monotherapy in patients for whom carboplatin rather than cisplatin was selected by the investigator as the better choice of chemotherapy were consistent with KEYNOTE-052 results. Efficacy results in patients whose tumours express PD-L1 with CPS ≥10 were similar to the overall population for whom carboplatin was s elected as the choice of chemotherapy. See Table 29 and Figures 25 and 26.
Table 29. Response to pembrolizumab 200 mg every 3 weeks or chemotherapy in patients with previously untreated urothelial carcinoma for whom carboplatin rather than cisplatin was selected by the investigator as the better choice of chemotherapy in KEYNOTE-361:
| Endpoint | Pembrolizumab n=170 | Chemotherapy n=196 | Pembrolizumab CPS ≥10 n=84 | Chemotherapy CPS ≥10 n=89 |
|---|---|---|---|---|
| Objective response rate* | ||||
| ORR % (95% CI) | 28% (21.1, 35.0) | 42% (34.8, 49.1) | 30% (20.3, 40.7) | 46% (35.4, 57.0) |
| Complete response | 10% | 11% | 12% | 18% |
| Partial response | 18% | 31% | 18% | 28% |
| Response duration* | ||||
| Median in months (range) | NR (3.2+, 36.1+) | 6.3 (1.8+, 33.8+) | NR (4.2, 36.1+) | 8.3 (2.1+, 33.8+) |
| % with duration ≥12 months† | 57% | 30% | 63% | 38% |
| PFS* | ||||
| Median in months (95% CI) | 3.2 (2.2, 5.5) | 6.7 (6.2, 8.1) | 3.9 (2.2, 6.8) | 7.9 (6.1, 9.3) |
| 12-month PFS rate | 25% | 24% | 26% | 31% |
| OS | ||||
| Median in months (95% CI) | 14.6 (10.2, 17.9) | 12.3 (10.0, 15.5) | 15.6 (8.6, 19.7) | 13.5 (9.5, 21.0) |
| 12-month OS rate | 54% | 51% | 57% | 54% |
* Assessed by BICR using RECIST 1.1
† Based on Kaplan-Meier estimation
NR = not reached
Figure 25. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-361 (intent to treat population, choice of carboplatin):
Figure 26. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-361 (patients with PD-L1 expression CPS ≥10, intent to treat population, choice of carboplatin):
Head and Neck Squamous Cell Carcinoma
KEYNOTE-689: Controlled study for the neoadjuvant and adjuvant treatment of patients with resectable locally advanced HNSCC
The efficacy of pembrolizumab was investigated in KEYNOTE-689, a randomised, multicentre, open-label, active-controlled study conducted in 714 patients with resectable locally advanced (Stage III-IVA) HNSCC. Patients with active autoimmune disease that required systemic therapy within two years of treatment or a medical condition that required immunosuppression were ineligible. Randomisation was stratified by primary tumour site (orophary nx/oral cavity vs. larynx vs. hypopharynx), tumour stage by AJCC 8th edition (III vs. IVA) and PD-L1 status (TPS ≥50% vs. TPS <50%).
Patients were randomised (1:1) to one of the following treatment arms:
- Treatment arm A: Neoadjuvant pembrolizumab 200 mg for 2 cycles prior to surgical resection. Within 6 weeks following surgery, pembrolizumab 200 mg for 3 cycles in combination with either radiation + 3 cycles of concomitant cisplatin 100 mg/m² every 3 weeks for patients with high-risk pathological features after surgery or radiation alone for patients without high-risk pathological features after surgery. This was followed by pembrolizumab 200 mg every 3 weeks for up to 12 cycles.
- Treatment arm B: No neoadjuvant treatment prior to surgery. Within 6 weeks following surgery, either radiation + 3 cycles of concomitant cisplatin 100 mg/m² every 3 weeks for patients with high-risk pathological features after surgery or radiation alone fo r patients without high-risk pathological features after surgery.
High-risk pathologic features are defined by evidence of positive margins or extranodal extension following surgical resection.
Treatment with pembrolizumab continued until RECIST v1.1-defined progression of disease as assessed by BICR, until completion of the treatment (17 cycles), disease progression that precluded definitive surgery, disease recurrence in the adjuvant phase, disease progression for those who did not undergo surgery or had incomplete resection and entered the adjuvant phase, or unacceptable toxicity. Assessment of tumour status was performed prior to surgery at Week 6 in the neoadjuvant phase. Following the start of the adjuvant phase, assessment of tumour status was perfo rmed 12 weeks after end of RT ± cisplatin treatment and then every 3 months until the end of Year 3; then every 6 months thereafter up to the end of Year 5. In arm A, 89% of the patients had surgery compared to 88% in arm B. In arm A, 29% of patients recei ved cisplatin plus radiation and 46% received radiation alone. In arm B, 40% of patients received cisplatin plus radiation and 39% received radiation alone.
Among the 714 patients in KEYNOTE-689, 682 (96 %) had tumours that expressed PD-L1 with a CPS ≥1 based on the PD -L1 IHC 22C3 pharmDxTM Kit. The baseline characteristics of these 682 patients were: median age of 60 years (range: 22 to 87), 33% age 65 or older; 79% male; 78% White, 13% Asian and 2.5% Black; 43% had ECOG PS of 1, and 79% were former/current smokers. Four percent of patients' tumours were HPV-positive, and 26% had Stage III disease, 74% had Stage IVA disease.
The primary efficacy outcome measure was EFS by BICR defined as the time from randomisation to the first occurrence of any of the following events: progression of disease that precludes definitive surgery, local or distant disease progression or recurrence, or death due to any cause. Secondary primary malignancy was not considered an event. Additional efficacy outcome measures were mPR as assessed by BIPR, OS, and pCR as assessed by BIPR.
The study demonstrated a statistically significant improvement in EFS (HR 0.73; 95% CI: 0.58, 0.92; p-Value 0.00411) in patients randomised to pembrolizumab in combination with radiation with or without concomitant cisplatin compared to those randomised to radiation with or without concomitant cisplatin at the first pre-specified interim analysis in the overall population. OS at interim analysis was not formally tested. Table 30 summarises key efficacy results for the pre-specified subgroup of patients whose tumours expressed PD-L1 with a CPS ≥1 at a median follow-up time of 27.0 months (range: 0.5 to 66.5 months). Figures 27 and 28 show the Kaplan-Meier curves for EFS and OS.
Table 30. Efficacy results in KEYNOTE-689 for patients with PD -L1 expression (CPS ≥1):
| Endpoint | Pembrolizumab 200 mg every 3 weeks with RT with or without cisplatin n=347 | RT with or without cisplatin n=335 |
|---|---|---|
| EFS | ||
| Number (%) of patients with event | 128 (37%) | 156 (47%) |
| Median in months* (95% CI) | 59.7 (37.9, NR) | 29.6 (19.5, 41.9) |
| Hazard ratio† (95% CI) | 0.70 (0.55, 0.89) | |
| p-Value‡ | 0.00140 | |
| OS | ||
| Number (%) of patients with event | 106 (31%) | 128 (38%) |
| Median in months* (95% CI) | NR (NR, NR) | 61.8 (49.2, NR) |
| Hazard ratio† (95% CI) | 0.72 (0.56, 0.94) | |
* From product-limit (Kaplan-Meier) method for censored data
† Based on Cox regression model with Efron's method of tie handling with treatment as a covariate stratified by primary tumour site and tumour stage
‡ One-sided p-Value based on log-rank test stratified by primary tumour site and tumour stage: Compared to a one-sided p-Value boundary of 0.0124
NR = not reached
Figure 27. Kaplan-Meier curve for event-free survival by treatment arm in KEYNOTE-689 for patients with PD-L1 expression (CPS ≥1):
Figure 28. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-689 for patients with PD-L1 expression (CPS ≥1):
KEYNOTE-048: Controlled study of monotherapy and combination therapy in HNSCC patients naïve to treatment in the recurrent or metastatic setting
The efficacy of pembrolizumab was investigated in KEYNOTE-048, a multicentre, randomised, open-label, active-controlled study in patients with histologically confirmed metastatic or recurrent HNSCC of the oral cavity, pharynx or larynx, who had not previously received systemic therapy for recurrent or metastatic disease and who were considered incurable by local therapies. Patients with nasopharyngeal carcinoma, active autoimmune disease that required systemic therapy within two years of treatment or a medical condition that required immunosuppression were ineligible for the study. Randomisation was stratified by tumour PD-L1 expression (TPS ≥50% or <50%), HPV status (positive or negative), and ECOG PS (0 vs. 1). Patients were randomised 1:1:1 to one of the following treatment arms:
- Pembrolizumab 200 mg every 3 weeks
- Pembrolizumab 200 mg every 3 weeks, carboplatin AUC 5 mg/mL/min every 3 weeks or cisplatin 100 mg/m² every 3 weeks, and 5-FU 1 000 mg/m²/d 4 days continuous every 3 weeks (maximum of 6 cycles of platinum and 5-FU)
- Cetuximab 400 mg/m² load then 250 mg/m² once weekly, carboplatin AUC 5 mg/mL/min every 3 weeks or cisplatin 100 mg/m² every 3 weeks, and 5-FU 1 000 mg/m²/d 4 days continuous every 3 weeks (maximum of 6 cycles of platinum and 5-FU)
Treatment with pembrolizumab continued until RECIST 1.1-defined progression of disease as determined by the investigator, unacceptable toxicity, or a maximum of 24 months. Administration of pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumour status was performed at Week 9 and then every 6 weeks for the first year, followed by every 9 weeks through 24 months.
Among the 882 patients in KEYNOTE-048, 754 (85%) had tumours that expressed PD-L1 with a CPS ≥1 based on the PD-L1 IHC 22C3 pharmDxTM Kit. The baseline characteristics of these 754 patients included: median age of 61 years (range: 20 to 94); 36% age 65 or older; 82% male; 74% White and 19% Asian; 61% ECOG performance status of 1; and 77% former/current smokers. Disease characteristics were: 21% HPV positive and 95% had stage IV disease (stage IVa 21%, stage IVb 6%, and stage IVc 69%).
The primary efficacy outcome measures were OS and PFS (assessed by BICR according to RECIST 1.1). The study demonstrated a statistically significant improvement in OS for all patients randomised to pembrolizumab in combination with chemotherapy compared to standard treatment (HR 0.72; 95% CI 0.60-0.87) and in patients whose tumours expressed PD-L1 CPS ≥1 randomised to pembrolizumab monotherapy compared to standard treatment. Tables 31 and 32 summarise key efficacy results for pembrolizumab in patients whose tumours expressed PD-L1 with a CPS ≥1 in KEYNOTE-048 at the final analysis performed at a median follow-up of 13 months for pembrolizumab in combination with chemotherapy and at a median follow-up of 11.5 months for pembrolizumab monotherapy. Kaplan-Meier curves for OS based on the final analysis are shown in Figures 29 and 30.
Table 31. Efficacy results for pembrolizumab plus chemotherapy in KEYNOTE-048 with PD-L1 expression (CPS ≥1):
| Endpoint | Pembrolizumab + Platinum Chemotherapy + 5-FU n=242 | Standard Treatment* n=235 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 177 (73%) | 213 (91%) |
| Median in months (95% CI) | 13.6 (10.7, 15.5) | 10.4 (9.1, 11.7) |
| Hazard ratio† (95% CI) | 0.65 (0.53, 0.80) | |
| p-Value‡ | 0.00002 | |
| PFS | ||
| Number (%) of patients with event | 212 (88%) | 221 (94%) |
| Median in months (95% CI) | 5.1 (4.7, 6.2) | 5.0 (4.8, 6.0) |
| Hazard ratio† (95% CI) | 0.84 (0.69, 1.02) | |
| p-Value‡ | 0.03697 | |
| Objective response rate | ||
| ORR§ % (95% CI) | 36% (30.3, 42.8) | 36% (29.6, 42.2) |
| Complete response | 7% | 3% |
| Partial response | 30% | 33% |
| p-Value¶ | 0.4586 | |
| Response duration | ||
| Median in months (range) | 6.7 (1.6+, 39.0+) | 4.3 (1.2+, 31.5+) |
| % with duration ≥6 months | 54% | 34% |
* Cetuximab, platinum, and 5-FU
† Based on the stratified Cox proportional hazard model
‡ Based on stratified log-rank test
§ Response: Best objective response as confirmed complete response or partial response
¶ Based on Miettinen and Nurminen method stratified by ECOG (0 vs. 1), HPV status (positive vs. negative) and PD-L1 status (strongly positive vs. not strongly positive)
Figure 29. Kaplan-Meier curve for overall survival for pembrolizumab plus chemotherapy in KEYNOTE-048 with PD-L1 expression (CPS ≥1):
Table 32. Efficacy results for pembrolizumab as monotherapy in KEYNOTE-048 with PD-L1 expression (CPS ≥1):
| Endpoint | Pembrolizumab n=257 | Standard Treatment* n=255 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 197 (77%) | 229 (90%) |
| Median in months (95% CI) | 12.3 (10.8, 14.3) | 10.3 (9.0, 11.5) |
| Hazard ratio† (95% CI) | 0.74 (0.61, 0.90) | |
| p-Value‡ | 0.00133 | |
| PFS | ||
| Number (%) of patients with event | 228 (89%) | 237 (93%) |
| Median in months (95% CI) | 3.2 (2.2, 3.4) | 5.0 (4.8, 6.0) |
| Hazard ratio† (95% CI) | 1.13 (0.94, 1.36) | |
| p-Value‡ | 0.89580 | |
| Objective response rate | ||
| ORR§ % (95% CI) | 19.1% (14.5, 24.4) | 35% (29.1, 41.1) |
| Complete response | 5% | 3% |
| Partial response | 14% | 32% |
| p-Value¶ | 1.0000 | |
| Response duration | ||
| Median in months (range) | 23.4 (1.5+, 43.0+) | 4.5 (1.2+, 38.7+) |
| % with duration ≥6 months | 81% | 36% |
* Cetuximab, platinum, and 5-FU
† Based on the stratified Cox proportional hazard model
‡ Based on stratified log-rank test
§ Response: Best objective response as confirmed complete response or partial response
¶ Based on Miettinen and Nurminen method stratified by ECOG (0 vs. 1), HPV status (positive vs. negative) and PD-L1 status (strongly positive vs. not strongly positive)
Figure 30. Kaplan-Meier curve for overall survival for pembrolizumab as monotherapy in KEYNOTE-048 with PD-L1 expression (CPS ≥1):
An analysis was performed in KEYNOTE-048 in patients whose tumours expressed PD-L1 CPS ≥20 [pembrolizumab plus chemotherapy: n=126 (49%) vs. standard treatment: n=110 (43%) and pembrolizumab monotherapy: n=133 (52%) vs. standard treatment: n=122 (48%)] (see Table 33).
Table 33. Efficacy results for pembrolizumab plus chemotherapy and pembrolizumab as monotherapy by PD-L1 expression in KEYNOTE-048 (CPS ≥20):
| Endpoint | Pembrolizumab + Platinum Chemotherapy + 5-FU n=126 | Standard Treatment* n=110 | Pembrolizumab Monotherapy n=133 | Standard Treatment* n=122 |
|---|---|---|---|---|
| OS | ||||
| Number (%) of patients with event | 84 (66.7%) | 98 (89.1%) | 94 (70.7%) | 108 (88.5%) |
| Median in months (95% CI) | 14.7 (10.3, 19.3) | 11.0 (9.2, 13.0) | 14.8 (11.5, 20.6) | 10.7 (8.8, 12.8) |
| Hazard ratio† (95% CI) | 0.60 (0.45, 0.82) | 0.58 (0.44, 0.78) | ||
| p-Value‡ | 0.00044 | 0.00010 | ||
| OS rate at 6 months (95% CI) | 74.6 (66.0, 81.3) | 80.0 (71.2, 86.3) | 74.4 (66.1, 81.0) | 79.5 (71.2, 85.7) |
| OS rate at 12 months (95% CI) | 57.1 (48.0, 65.2) | 46.1 (36.6, 55.1) | 56.4 (47.5, 64.3) | 44.9 (35.9, 53.4) |
| OS rate at 24 months (95% CI) | 35.4 (27.2, 43.8) | 19.4 (12.6, 27.3) | 35.3 (27.3, 43.4) | 19.1 (12.7, 26.6) |
| PFS | ||||
| Number (%) of patients with event | 106 (84.1%) | 104 (94.5%) | 115 (86.5%) | 114 (93.4%) |
| Median in months (95% CI) | 5.8 (4.7, 7.6) | 5.3 (4.9, 6.3) | 3.4 (3.2, 3.8) | 5.3 (4.8, 6.3) |
| Hazard ratio† (95% CI) | 0.76 (0.58, 1.01) | 0.99 (0.76, 1.29) | ||
| p-Value‡ | 0.02951 | 0.46791 | ||
| PFS rate at 6 months (95% CI) | 49.4 (40.3, 57.9) | 47.2 (37.5, 56.2) | 33.0 (25.2, 41.0) | 46.6 (37.5, 55.2) |
| PFS rate at 12 months (95% CI) | 23.9 (16.7, 31.7) | 14.0 (8.2, 21.3) | 23.5 (16.6, 31.1) | 15.1 (9.3, 22.2) |
| PFS rate at 24 months (95% CI) | 14.6 (8.9, 21.5) | 5.0 (1.9, 10.5) | 16.8 (10.9, 23.8) | 6.1 (2.7, 11.6) |
| Objective response rate | ||||
| ORR§ % (95% CI) | 42.9 (34.1, 52.0) | 38.2 (29.1, 47.9) | 23.3 (16.4, 31.4) | 36.1 (27.6, 45.3) |
| Response duration | ||||
| Number of responders | 54 | 42 | 31 | 44 |
| Median in months (range) | 7.1 (2.1+, 39.0+) | 4.2 (1.2+, 31.5+) | 22.6 (2.7+, 43.0+) | 4.2 (1.2+, 31.5+) |
* Cetuximab, platinum, and 5-FU
† Based on the stratified Cox proportional hazard model
‡ Based on stratified log-rank test
§ Response: Best objective response as confirmed complete response or partial response
An exploratory subgroup analysis was performed in KEYNOTE-048 in patients whose tumours expressed PD-L1 CPS ≥1 to <20 [pembrolizumab plus chemotherapy: n=116 (45%) vs. standard treatment: n=125 (49%) and pembrolizumab monotherapy: n=124 (48%) vs. standard treatment: n=133 (52%)] (see Table 34).
Table 34. Efficacy results for pembrolizumab plus chemotherapy and pembrolizumab as monotherapy by PD-L1 expression in KEYNOTE-048 (CPS ≥1 to <20):
| Endpoint | Pembrolizumab + Platinum Chemotherapy + 5-FU n=116 | Standard Treatment* n=125 | Pembrolizumab Monotherapy n=124 | Standard Treatment* n=133 |
|---|---|---|---|---|
| OS | ||||
| Number (%) of patients with event | 93 (80.2%) | 115 (92.0%) | 103 (83.1%) | 121 (91.0%) |
| Median in months (95% CI) | 12.7 (9.4, 15.3) | 9.9 (8.6, 11.5) | 10.8 (9.0, 12.6) | 10.1 (8.7, 12.1) |
| Hazard ratio† (95% CI) | 0.71 (0.54, 0.94) | 0.86 (0.66, 1.12) | ||
| OS rate at 6 months (95% CI) | 76.7 (67.9, 83.4) | 77.4 (69.0, 83.8) | 67.6 (58.6, 75.1) | 78.0 (70.0, 84.2) |
| OS rate at 12 months (95% CI) | 52.6 (43.1, 61.2) | 41.1 (32.4, 49.6) | 44.0 (35.1, 52.5) | 42.4 (33.9, 50.7) |
| OS rate at 24 months (95% CI) | 25.9 (18.3, 34.1) | 14.5 (9.0, 21.3) | 22.0 (15.1, 29.6) | 15.9 (10.3, 22.6) |
| PFS | ||||
| Number (%) of patients with event | 106 (91.4%) | 117 (93.6%) | 113 (91.1%) | 123 (92.5%) |
| Median in months (95% CI) | 4.9 (4.2, 5.3) | 4.9 (3.7, 6.0) | 2.2 (2.1, 2.9) | 4.9 (3.8, 6.0) |
| Hazard ratio† (95% CI) | 0.93 (0.71, 1.21) | 1.25 (0.96, 1.61) | ||
| PFS rate at 6 months (95% CI) | 40.1 (31.0, 49.0) | 40.0 (31.2, 48.5) | 24.2 (17.1, 32.0) | 41.4 (32.8, 49.7) |
| PFS rate at 12 months (95% CI) | 15.1 (9.1, 22.4) | 11.3 (6.4, 17.7) | 17.5 (11.4, 24.7) | 12.1 (7.2, 18.5) |
| PFS rate at 24 months (95% CI) | 8.5 (4.2, 14.7) | 5.0 (1.9, 10.1) | 8.3 (4.3, 14.1) | 6.3 (2.9, 11.5) |
| Objective response rate | ||||
| ORR‡ % (95% CI) | 29.3 (21.2, 38.5) | 33.6 (25.4, 42.6) | 14.5 (8.8, 22.0) | 33.8 (25.9,42.5) |
| Response duration | ||||
| Number of responders | 34 | 42 | 18 | 45 |
| Median in months (range) | 5.6 (1.6+, 25.6+) | 4.6 (1.4+, 31.4+) | NR (1.5+, 38.9+) | 5.0 (1.4+, 38.7+) |
* Cetuximab, platinum, and 5-FU
† Based on the stratified Cox proportional hazard model
‡ Response: Best objective response as confirmed complete response or partial response
NR = not reached
KEYNOTE-040: Controlled study in HNSCC patients previously treated with platinum-containing chemotherapy
The safety and efficacy of pembrolizumab were investigated in KEYNOTE-040, a multicentre, open-label, randomised, controlled study for the treatment of histologically confirmed recurrent or metastatic HNSCC of the oral cavity, pharynx or larynx in patients who had disease progression on or after platinum-containing chemotherapy administered for recurrent or metastatic HNSCC or following platinum-containing chemotherapy administered as part of induction, concurrent, or adjuvant therapy, and were not amenable to local therapy with curative intent. Patients were stratified by PD-L1 expression (TPS ≥50%), HPV status and ECOG performance status and then randomised (1:1) to receive either pembrolizumab 200 mg every 3 weeks (n=247) or one of three standard treatments (n=248): methotrexate 40 mg/m² once weekly (n=64), docetaxel 75 mg/m² once every 3 weeks (n=99), or cetuximab 400 mg/m² loading dose and then 250 mg/m² once weekly (n=71). Treatment could continue beyond progression if the patient was clinically stable and was considered to be deriving clinical benefit by the investigator. The study excluded patients with nasopharyngeal carcinoma, active autoimmune disease that required systemic therapy within 2 years of treatment, a medical condition that required immunosuppression, or who were previously treated with 3 or more systemic regimens for recurrent and/or metastatic HNSCC. Assessment of tumour status was performed at 9 weeks, then every 6 weeks through Week 52, followed by every 9 weeks through 24 months.
Among the 495 patients in KEYNOTE-040, 129 (26%) had tumours that expressed PD-L1 with a TPS ≥50% based on the PD-L1 IHC 22C3 pharmDxTM Kit. The baseline characteristics of these 129 patients included: median age 62 years (40% age 65 or older); 81% male; 78% White, 11% Asian, and 2% Black; 23% and 77% with an ECOG performance status 0 or 1, respectively; and 19% with HPV positive tumours. Sixty-seven percent (67%) of patients had M1 disease and the majority had stage IV disease (stage IV 32%, stage IVa 14%, stage IVb 4%, and stage IVc 44%). Sixteen percent (16%) had disease progression following plati num-containing neoadjuvant or adjuvant chemotherapy, and 84% had received 1-2 prior systemic regimens for metastatic disease.
The primary efficacy outcome was OS in the ITT population. The initial analysis resulted in a HR for OS of 0.82 (95% CI: 0.67, 1.01) with a one-sided p-Value of 0.0316. The median OS was 8.4 months for pembrolizumab compared to 7.1 months for standard treatment. Table 35 summarises the key efficacy measures for the TPS ≥50% population. The Kaplan-Meier curve for OS for the TPS ≥50% population is shown in Figure 31.
Table 35. Efficacy of pembrolizumab 200 mg every 3 weeks in HNSCC patients with TPS ≥50% who were previously treated with platinum chemotherapy in KEYNOTE-040:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=64 | Standard Treatment* n=65 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 41 (64%) | 56 (86%) |
| Hazard ratio† (95% CI) | 0.53 (0.35, 0.81) | |
| p-Value‡ | 0.001 | |
| Median in months (95% CI) | 11.6 (8.3, 19.5) | 6.6 (4.8, 9.2) |
| PFS§ | ||
| Number (%) of patients with event | 52 (81%) | 58 (89%) |
| Hazard ratio† (95% CI) | 0.58 (0.39, 0.86) | |
| p-Value‡ | 0.003 | |
| Median in months (95% CI) | 3.5 (2.1, 6.3) | 2.1 (2.0, 2.4) |
| Rate (%) at 6 months (95% CI) | 40.1 (28.1, 51.9) | 17.1 (8.8, 27.7) |
| Objective response rate§ | ||
| ORR % (95% CI) | 26.6 (16.3, 39.1) | 9.2 (3.5, 19.0) |
| p-Value¶ | 0.0009 | |
| Complete response | 5% | 2% |
| Partial response | 22% | 8% |
| Stable disease | 23% | 23% |
| Response duration§,# | ||
| Median in months (range) | Not reached (2.7, 13.8+) | 6.9 (4.2, 18.8) |
| Number (%Þ) of patients with duration ≥6 months | 9 (66%) | 2 (50%) |
* Methotrexate, docetaxel, or cetuximab
† Hazard ratio (pembrolizumab compared to standard treatment) based on the stratified Cox proportional hazard model
‡ One-sided p-Value based on log-rank test
§ Assessed by BICR using RECIST 1.1
¶ Based on method by Miettinen and Nurminen
# Based on patients with a best objective response as confirmed complete or partial response
Þ Based on Kaplan-Meier estimation
Figure 31. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-040 patients with PD-L1 expression (TPS ≥50%):
Renal cell carcinoma
KEYNOTE-426: Controlled study of combination therapy with axitinib in RCC patients naïve to treatment
The efficacy of pembrolizumab in combination with axitinib was investigated in KEYNOTE-426, a randomised, multicentre, open-label, active-controlled study conducted in patients with advanced RCC with clear cell component, regardless of PD-L1 tumour expression status and International Metastatic RCC Database Consortium (IMDC) risk group categories. The study excluded patients with autoimmune disease or a medical condition that required immunosuppression. Randomisation was stratified by risk categories (favourable versus intermediate versus poor) and geographic region (North America versus Western Europe versus "Rest of the World"). Patients were randomised (1:1) to one of the following treatment arms:
- pembrolizumab 200 mg intravenously every 3 weeks in combination with axitinib 5 mg orally, twice daily. Patients who tolerated axitinib 5 mg twice daily for 2 consecutive treatment cycles (i.e. 6 weeks) with no > Grade 2 treatment-related adverse events to axitinib and with blood pressure well controlled to ≤150/90 mm Hg were permitted dose escalation of axitinib to 7 mg twice daily. Dose escalation of axitinib to 10 mg twice daily was permitted using the same criteria. Axitinib could be interrupted or reduced to 3 mg twice daily and subsequently to 2 mg twice daily to manage toxicity.
- sunitinib 50 mg orally, once daily for 4 weeks and then off treatment for 2 weeks.
Treatment with pembrolizumab and axitinib continued until RECIST v1.1-defined progression of disease as verified by BICR or confirmed by the investigator, unacceptable toxicity, or for pembrolizumab, a maximum of 24 months. Administration of pembrolizumab and axitinib was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumour status was performed at baseline, after randomisation at Week 12, then every 6 weeks thereafter until Week 54, and then every 12 weeks thereafter.
A total of 861 patients were randomised. The study population characteristics were: median age of 62 years (range: 26 to 90); 38% age 65 or older; 73% male; 79% White and 16% Asian; 80% had a Karnofsky Performance Score (KPS) 90-100 and 20% had KPS 70-80; patient distribution by IMDC risk categories was 31% favourable, 56% intermediate and 13% poor.
The primary efficacy outcome measures were OS and PFS (as assessed by BICR using RECIST 1.1). Secondary efficacy outcome measures were ORR and response duration, as assessed by BICR using RECIST 1.1. The study demonstrated a statistically significant improvement in OS (HR 0.53; 95% CI 0.38, 0.74; p-Value = 0.00005) and PFS (HR 0.69; 95% CI 0.56, 0.84; p-Value = 0.00012) for patients randomised to the pembrolizumab combination arm compared with sunitinib at its pre-specified interim analysis. Table 36 summarises key efficacy measures and Figures 32 and 33 show the Kaplan-Meier curves for OS and PFS based on the final analysis with a median follow-up time of 37.7 months.
Table 36. Efficacy results in KEYNOTE-426:
| Endpoint | Pembrolizumab Axitinib n=432 | Sunitinib n=429 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 193 (45%) | 225 (52%) |
| Median in months (95% CI) | 45.7 (43.6, NA) | 40.1 (34.3, 44.2) |
| Hazard ratio* (95% CI) | 0.73 (0.60, 0.88) | |
| p-Value† | 0.00062 | |
| PFS‡ | ||
| Number (%) of patients with event | 286 (66%) | 301 (70%) |
| Median in months (95% CI) | 15.7 (13.6, 20.2) | 11.1 (8.9, 12.5) |
| Hazard ratio* (95% CI) | 0.68 (0.58, 0.80) | |
| p-Value† | <0.00001 | |
| Objective response rate | ||
| ORR§ % (95% CI) | 60 (56, 65) | 40 (35, 44) |
| Complete response | 10% | 3% |
| Partial response | 50% | 36% |
| p-Value¶ | <0.0001 | |
| Response duration | ||
| Median in months (range) | 23.6 (1.4+, 43.4+) | 15.3 (2.3, 42.8+) |
| Number (%#) of patients with duration ≥30 months | 87 (45%) | 29 (32%) |
* Based on the stratified Cox proportional hazard model
† Nominal p-Value based on stratified log-rank test
‡ Assessed by BICR using RECIST 1.1
§ Based on patients with a best objective response as confirmed complete or partial response
¶ Nominal p-Value based on Miettinen and Nurminen method stratified by IMDC risk group and geographic region. At the pre-specified interim analysis of ORR (median follow-up time of 12.8 months), statistically significant superiority was achieved for ORR comparing pembrolizumab plus axitinib with sunitinib p-Value <0.0001.
# Based on Kaplan-Meier estimation
NA = not available
Figure 32. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-426 (intent to treat population):
Figure 33. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-426 (intent to treat population):
Subgroup analyses were performed in KEYNOTE-426 in patients with PD-L1 CPS ≥1 [pembrolizumab/axitinib combination: n=243 (56%) vs. sunitinib: n=254 (59%)] and CPS <1 [pembrolizumab/axitinib combination: n=167 (39%) vs. sunitinib: n=158 (37%)]. OS and PFS benefits were observed regardless of PD-L1 expression level.
The KEYNOTE-426 study was not powered to evaluate efficacy of individual subgroups.
Table 37 summarises the efficacy measures by IMDC risk category based on the final OS analysis at a median follow-up of 37.7 months.
Table 37. Efficacy results in KEYNOTE-426 by IMDC risk category:
| Endpoint* | Pembrolizumab + Axitinib n=432 | Sunitinib n=429 | Pembrolizumab + Axitinib vs. Sunitinib |
|---|---|---|---|
| OS | 12-month OS rate, % (95% CI) | OS HR (95% CI) | |
| Favourable | 95.6 (90.5, 98.0) | 94.6 (89.0, 97.4) | 1.17 (0.76, 1.80) |
| Intermediate | 90.7 (86.2, 93.8) | 77.6 (71.8, 82.3) | 0.67 (0.52, 0.86) |
| Poor | 69.6 (55.8, 79.9) | 45.1 (31.2, 58.0) | 0.51 (0.32, 0.81) |
| PFS | Median (95% CI), months | PFS HR (95% CI) | |
| Favourable | 20.7 (15.2, 28.9) | 17.8 (12.5, 20.7) | 0.76 (0.56, 1.03) |
| Intermediate | 15.3 (12.5, 20.8) | 9.7 (8.0, 12.4) | 0.69 (0.55, 0.86) |
| Poor | 4.9 (2.8, 12.4) | 2.9 (2.7, 4.2) | 0.53 (0.33, 0.84) |
| Confirmed ORR | % (95% CI) | ORR difference, % (95% CI) | |
| Favourable | 68.8 (60.4, 76.4) | 50.4 (41.5, 59.2) | 18.5 (6.7, 29.7) |
| Intermediate | 60.5 (54.0, 66.8) | 39.8 (33.7, 46.3) | 20.7 (11.8, 29.2) |
| Poor | 39.3 (26.5, 53.2) | 11.5 (4.4, 23.4) | 27.7 (11.7, 42.8) |
* n (%) for favourable, intermediate and poor risk categories for pembrolizumab/axitinib vs. sunitinib were: 138 (32%) vs. 131 (31%); 238 (55%) vs. 246 (57%); 56 (13%) vs. 52 (12%), respectively
KEYNOTE-581 (CLEAR): Controlled study of combination therapy with lenvatinib in RCC patients naïve to treatment
The efficacy of pembrolizumab in combination with lenvatinib was investigated in KEYNOTE-581 (CLEAR), a multicentre, open-label, randomised study conducted in 1 069 patients with advanced RCC with clear cell component including other histological features such as sarcomatoid and papillary in the first-line setting. Patients were enrolled regardless of PD-L1 tumour expression status. The study excluded patients with active autoimmune disease or a medical condition that required immunosuppression. Randomisation was stratified by geographic region (North America versus Western Europe versus "Rest of the World") and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic groups (favourable versus intermediate versus poor).
Patients were randomised (1:1:1) to one of the following treatment arms:
- pembrolizumab 200 mg intravenously every 3 weeks up to 24 months in combination with lenvatinib 20 mg orally once daily.
- lenvatinib 18 mg orally once daily in combination with everolimus 5 mg orally once daily.
- sunitinib 50 mg orally once daily for 4 weeks then off treatment for 2 weeks.
Treatment continued until unacceptable toxicity or disease progression as determined by the investigator and confirmed by BICR using RECIST 1.1. Administration of pembrolizumab with lenvatinib was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered by the investigator to be deriving clin ical benefit. Pembrolizumab was continued for a maximum of 24 months; however, treatment with lenvatinib could be continued beyond 24 months. Assessment of tumour status was performed at baseline and then every 8 weeks.
Among the study population (355 patients in the pembrolizumab with lenvatinib arm and 357 in the sunitinib arm), the baseline characteristics were: median age of 62 years (range: 29 to 88 years), 41% age 65 or older; 74% male; 75% White, 21% Asian, 1% Black, and 2% other races; 17% and 83% of patients had a baseline KPS of 70 to 80 and 90 to 100, respectively; patient distribution by IMDC risk categories was 33% favourable, 56% intermediate and 10% poor, and by MSKCC prognostic groups was 27% favourable, 64% intermediate and 9% poor. Metastatic disease was present in 99% of the patients and locally advanced disease was present in 1%. Common sites of metastases in patients were lung (69%), lymph node (46%), and bone (26%).
The primary efficacy outcome measure was PFS based on BICR using RECIST 1.1. Key secondary efficacy outcome measures included OS and ORR. The study demonstrated statistically significant improvements in PFS (HR 0.39; 95% CI 0.32, 0.49; p-Value <0.0001), OS (HR 0.66; 95% CI 0.49, 0.88; p-Value 0.0049), and ORR (71%; [95% CI 66, 76] vs. 36%; [95% CI 31, 41]; p-Value <0.0001) in patients randomised to pembrolizumab in combination with lenvatinib compared with sunitinib at the pre-specified interim analysis, with a median survival follow-up time of 26.5 months, and median duration of treatment for pembrolizumab plus lenvatinib of 17.0 months. The primary OS analysis was not adjusted to account for subsequent therapies.
Efficacy results for KEYNOTE-581 (CLEAR) at the protocol-specified final analysis with median follow-up time of 49.4 months are summarised in Table 38 and Figures 34 and 35. PFS results were consistent across pre-specified subgroups, MSKCC prognostic groups and PD-L1 tumour expression status. Efficacy results by MSKCC prognostic group are summarised in Table 39.
Table 38. Efficacy results in KEYNOTE-581 (CLEAR):
| Endpoint | Pembrolizumab 200 mg every 3 weeks and Lenvatinib n=355 | Sunitinib n=357 |
|---|---|---|
| PFS* | ||
| Number (%) of patients with event | 207 (58%) | 214 (60%) |
| Median in months (95% CI) | 23.9 (20.8, 27.7) | 9.2 (6.0, 11.0) |
| Hazard ratio† (95% CI) | 0.47 (0.38, 0.57) | |
| p-Value‡ | <0.0001 | |
| OS | ||
| Number (%) of patients with event | 149 (42%) | 159 (45%) |
| Median in months (95% CI) | 53.7 (48.7, NR) | 54.3 (40.9, NR) |
| Hazard ratio† (95% CI) | 0.79 (0.63, 0.99) | |
| p-Value‡ | 0.0424 | |
| Objective response rate | ||
| ORR§ % (95% CI) | 71% (66.6, 76.0) | 37% (31.7, 41.7) |
| Complete response | 18% | 5% |
| Partial response | 53% | 32% |
| p-Value¶ | <0.0001 | |
| Response duration# | ||
| Median in months (range) | 26.7 (1.64+, 55.92+) | 14.7 (1.64+, 54.08+) |
* The primary analysis of PFS included censoring for new anti-cancer treatment. Results for PFS with and without censoring for new anti-cancer treatment were consistent.
† Based on the stratified Cox proportional hazard model
‡ Nominal p-Value, two-sided based on stratified log-rank test
§ Response: Best objective response as confirmed complete response or partial response
¶ Nominal two-sided p-Value based on the stratified Cochran-Mantel-Haenszel (CMH) test. At the earlier pre-specified final analysis of ORR (median follow-up time of 17.3 months), statistically significant superiority was achieved for ORR comparing pembrolizumab plus lenvatinib with sunitinib, (odds ratio: 3.84 [95% CI: 2.81, 5.26], p-Value <0.0001).
# Based on Kaplan-Meier estimates
NR = not reached
The final OS analysis was not adjusted to account for subsequent therapies, with 195/357 (54.6%) patients in the sunitinib arm and 56/355 (15.8%) patients in the pembrolizumab plus lenvatinib arm receiving subsequent anti-PD-1/PD-L1 therapy.
Figure 34. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-581 (CLEAR):
Figure 35. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-581 (CLEAR):
The KEYNOTE-581 (CLEAR) study was not powered to evaluate efficacy of individual subgroups.
Subgroup analyses were performed by MSKCC prognostic group.
Table 39 summarises the efficacy measures by MSKCC prognostic group based on the final OS analysis at a median follow-up of 49.4 months.
Table 39. Efficacy results in KEYNOTE-581 (CLEAR) by MSKCC prognostic group:
| Pembrolizumab + Lenvatinib (n=355) | Sunitinib (n=357) | Pembrolizumab + Lenvatinib vs. Sunitinib | |||
|---|---|---|---|---|---|
| Number of Patients | Number of Events | Number of Patients | Number of Events | ||
| Progression-Free Survival (PFS) by BICR* | PFS HR (95% CI) | ||||
| Favourable | 96 | 56 | 97 | 65 | 0.46 (0.32, 0.67) |
| Intermediate | 227 | 129 | 228 | 130 | 0.51 (0.40, 0.65) |
| Poor | 32 | 22 | 32 | 19 | 0.18 (0.08, 0.42) |
| Overall Survival (OS)* | OS HR (95% CI) | ||||
| Favourable | 96 | 27 | 97 | 31 | 0.89 (0.53, 1.50) |
| Intermediate | 227 | 104 | 228 | 108 | 0.81 (0.62, 1.06) |
| Poor | 32 | 18 | 32 | 20 | 0.59 (0.31, 1.12) |
* Median follow-up: 49.4 months (data cutoff – 31 July 2022)
KEYNOTE-B61: Open-label single arm Phase II study
Additional data are available from the open-label single arm Phase II study KEYNOTE-B61 of pembrolizumab (400 mg every 6 weeks) in combination with lenvatinib (20 mg OD) for the first-line treatment of patients with advanced or metastatic RCC with non-clear cell histology (n=158), including 59% papillary, 18% chromophobe, 4% translocation, 1% medullary, 13% unclassified, and 6% other. The ORR was 50.6% (95% CI: 42.6, 58.7) and the median duration of response was 19.5 months (95% CI: 15.3, NR).
KEYNOTE-564: Placebo-controlled study for the adjuvant treatment of patients with resected RCC
The efficacy of pembrolizumab was investigated as adjuvant therapy for RCC in KEYNOTE-564, a multicentre, randomised, double-blind, placebo-controlled study in 994 patients with increased risk of recurrence defined as intermediate-high or high risk, or M1 with no evidence of disease (NED). The intermediate-high risk category included: pT2 with Grade 4 or sarcomatoid features; pT3, any Grade without nodal involvement (N0) or distant metastases (M0). The high risk category included: pT4, any Grade N0 and M0; any pT, any Grade with nodal involvement and M0. The M1 NED category included patients with metastatic disease who had undergone complete resection of primary and metastatic lesions. Patients must have undergone a partial nephroprotective or radical comple te nephrectomy (and complete resection of solid, isolated, soft tissue metastatic lesion(s) in M1 NED participants) with negative surgical margins ≥4 weeks prior to the time of screening. The study excluded patients with active autoimmune disease or a medical condition that required immunosuppression. Patients with RCC with clear cell component were randomised (1:1) to receive pembrolizumab 200 mg every 3 weeks (n=496) or placebo (n=498) for up to 1 year until disease recurrence or unacceptable toxicity. Randomisation was stratified by metastasis status (M0, M1 NED), and within M0 group, further stratified by ECOG PS (0,1), and geographic region (US, non-US). Starting from randomisation, patients underwent imaging every 12 weeks for the first 2 years, then every 16 weeks from year 3 to 5, and then every 24 weeks annually.
Among the 994 patients, the baseline characteristics were: median age of 60 years (range: 25 to 84), 33% age 65 or older; 71% male; and 85% ECOG PS of 0 and 15% ECOG PS of 1. Ninety-four percent were N0; 83% had no sarcomatoid features; 86% were pT2 with Grade 4 or sarcomatoid features or pT3; 8% were pT4 or with nodal involvement; and 6% were M1 NED. Baseline characteristics and demographics were generally comparable between the pembrolizumab and placebo arms.
The primary efficacy outcome measure was investigator-assessed disease-free survival (DFS). The key secondary outcome measure was OS. The study demonstrated statistically significant improvements in DFS and OS for patients randomised to the pembrolizumab arm compared with placebo . At the pre-specified interim analysis with a median follow-up time of 23.9 months, the DFS HR was 0.68 (95% CI 0.53, 0.87; p-Value = 0.0010). Efficacy results with a median follow-up time of 55.8 months are summarised in Table 40 and Figures 36 and 37.
Table 40. Efficacy results in KEYNOTE-564:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=496 | Placebo n=498 |
|---|---|---|
| DFS | ||
| Number (%) of patients with event | 174 (35%) | 224 (45%) |
| Median in months (95% CI) | NR (NR, NR) | NR (54.9, NR) |
| Hazard ratio* (95% CI) | 0.72 (0.59, 0.87) | |
| OS | ||
| Number (%) of patients with event | 55 (11%) | 86 (17%) |
| Median in months (95% CI) | NR (NR, NR) | NR (NR, NR) |
| Hazard ratio* (95% CI) | 0.62 (0.44, 0.87) | |
| p-Value† | 0.0024 | |
* Based on the stratified Cox proportional hazard model
† One-sided p-Value based on stratified log-rank test
NR = not reached
Figure 36. Kaplan-Meier curve for disease-free survival by treatment arm in KEYNOTE-564 (intent to treat population):
Figure 37. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-564 (intent to treat population):
MSI-H or dMMR cancers
Colorectal cancer
KEYNOTE-177: Controlled study in MSI-H or dMMR CRC patients naïve to treatment in the metastatic setting
The efficacy of pembrolizumab was investigated in KEYNOTE-177, a multicentre, randomised, open-label, active-controlled study that enrolled patients wi th previously untreated metastatic MSI-H or dMMR CRC. MSI or MMR (mismatch repair) tumour status was determined locally using polymerase chain reaction (PCR) or IHC, respectively. Patients with autoimmune disease or a medical condition that required immuno suppression were ineligible.
Patients were randomised (1:1) to receive pembrolizumab 200 mg intravenously every 3 weeks or investigator's choice of the following chemotherapy regimens given intravenously every 2 weeks:
- mFOLFOX6 (oxaliplatin, leucovorin, and FU) or mFOLFOX6 in combination with either bevacizumab or cetuximab: Oxaliplatin 85 mg/m², leucovorin 400 mg/m² (or levoleucovorin 200 mg/m²), and FU 400 mg/m² bolus on Day 1, then FU 2 400 mg/m² over 46-48 hours. Bevacizumab 5 mg/kg bw on Day 1 or cetuximab 400 mg/m² on first infusion, then 250 mg/m² weekly.
- FOLFIRI (irinotecan, leucovorin, and FU) or FOLFIRI in combination with either bevacizumab or cetuximab: Irinotecan 180 mg/m², leucovorin 400 mg/m² (or levoleucovorin 200 mg/m²), and FU 400 mg/m² bolus on Day 1, then FU 2 400 mg/m² over 46-48 hours. Bevacizumab 5 mg/kg bw on Day 1 or cetuximab 400 mg/m² on first infusion, then 250 mg/m² weekly.
Treatment with pembrolizumab continued until RECIST v1.1-defined progression of disease as determined by the investigator or unacceptable toxicity. Patients treated with pembrolizumab without disease progression could be treated for up to 24 months. Assessment of tumour status was performed every 9 weeks. Patients randomised to chemotherapy were offered pembrolizumab at the time of disease progression.
A total of 307 patients were enrolled and randomised to pembrolizumab (n=153) or chemotherapy (n=154). The baseline characteristics of these patients were: medi an age of 63 years (range: 24 to 93), 47% age 65 or older; 50% male; 75% White and 16% Asian; 52% and 48% had an ECOG performance status of 0 or 1, respectively. Mutation status: 25% BRAF V600E, 24% KRAS/NRAS. For 143 patients treated with chemotherapy, 56% received mFOLFOX6 with or without bevacizumab or cetuximab and 44% received FOLFIRI with or without bevacizumab or cetuximab.
The primary efficacy outcome measures were PFS assessed by BICR according to RECIST v1.1 and OS. Secondary outcome measures were ORR and response duration. The study demonstrated a statistically significant improvement in PFS (HR 0.60; 95% CI 0.45, 0.80; p-Value 0.0002) for patients randomised to the pembrolizumab arm compared with chemotherapy at the pre-specified final analysis for PFS. There was no statistically significant difference between pembrolizumab and chemotherapy in the final OS analysis in which 60% of the patients who had been randomi sed to receive chemotherapy had crossed over to receive subsequent anti-PD-1/PD-L1 therapies including pembrolizumab. Table 40 summarises the key efficacy measures and Figures 38 and 39 show the Kaplan-Meier curves for updated PFS and OS based on the final analysis with a median follow-up time of 38.1 months (range: 0.2 to 58.7 months).
Table 41. Efficacy results in KEYNOTE-177:
| Endpoint | Pembrolizumab 200 mg every 3 weeks n=153 | Chemotherapy n=154 |
|---|---|---|
| PFS* | ||
| Number (%) of patients with event | 86 (56%) | 117 (76%) |
| Median in months (95% CI) | 16.5 (5.4, 38.1) | 8.2 (6.1, 10.2) |
| Hazard ratio† (95% CI) | 0.59 (0.45, 0.79) | |
| p-Value‡ | 0.0001 | |
| OS§ | ||
| Number (%) of patients with event | 62 (41%) | 78 (51%) |
| Median in months (95% CI) | NR (49.2, NR) | 36.7 (27.6, NR) |
| Hazard ratio† (95% CI) | 0.74 (0.53, 1.03) | |
| p-Value§ | 0.0359 | |
| Objective response rate | ||
| ORR % (95% CI) | 45% (37.1, 53.3) | 33% (25.8, 41.1) |
| Complete response | 13% | 4% |
| Partial response | 32% | 29% |
| Response duration | ||
| Median in months (range) | NR (2.3+, 53.5+) | 10.6 (2.8, 48.3+) |
| % with duration ≥24 months¶ | 84% | 34% |
* With additional 12 months of follow-up after the pre-specified final analysis for PFS.
† Based on Cox regression model
‡ p-Value is nominal.
§ Not statistically significant after adjustment for multiplicity
¶ Based on Kaplan-Meier estimation
NR = not reached
Figure 38. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-177 (intent to treat population):
Figure 39. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-177 (intent to treat population):
* Not statistically significant after adjustment for multiplicity
KEYNOTE-164: Open-label study in patients with unresectable or metastatic MSI-H or dMMR CRC who have received prior therapy
The efficacy of pembrolizumab was investigated in KEYNOTE-164, a multicentre, non-randomised, open-label, multi-cohort Phase II study that enrolled patients with unresectable or metastatic MSI-H or dMMR CRC that progressed following prior fluoropyrimidine-based therapy in combination with irinotecan and/or oxaliplatin.
Patients received pembrolizumab 200 mg every 3 weeks until unacceptable toxicity or disease progression. Clinically stable patients with initial evidence of disease progression were permitted to remain on treatment until disease progression was confirmed. Patients without disease progression were treated for up to 24 months (up to 35 cycles). Assessment of tumour status was performed every 9 weeks.
Among the 124 patients enrolled in KEYNOTE-164, the baseli ne characteristics were: median age 56 years (35% age 65 or older); 56% male; 68% White, 27% Asian; 41% and 59% had an ECOG performance status of 0 and 1, respectively. Twelve percent of patients had BRAF mutations and 36% had RAS mutations; 39% and 34% we re undetermined for BRAF and RAS mutations, respectively.
Ninety-seven percent of the patients had M1 disease and 3% had M0 disease (locally advanced unresectable). Seventy-six percent of patients received 2 or more prior lines of therapy.
The primary efficacy outcome measure was ORR as assessed by BICR using RECIST 1.1. Secondary efficacy outcome measures included response duration, PFS, and OS. The median follow-up time in months was 37.3 (range: 0.1 to 65.2). Efficacy results are summarised in Table 42.
Table 42. Efficacy results in KEYNOTE-164:
| Endpoint | n=124 |
|---|---|
| Objective response rate* | |
| ORR % (95% CI) | 34% (25.6, 42.9) |
| Complete response | 10% |
| Partial response | 24% |
| Response duration* | |
| Median in months (range) | NR (4.4, 58.5+) |
| % with duration ≥36 months# | 92% |
* Based on patients with a best objective response as confirmed complete or
partial response
# Based on Kaplan-Meier estimation
+ Denotes there is no progressive disease by the time of last disease assessment
NR = not reached
Objective responses were observed regardless of BRAF or RAS mutation status.
Non-colorectal cancers
KEYNOTE-158: Open-label study in patients with unresectable or metastatic MSI-H or dMMR endometrial, gastric, small intestine, or biliary cancer who have received prior therapy
The efficacy of pembrolizumab was investigated in 377 patients with unresectable or metastatic MSI-H or dMMR non-CRC solid tumours enrolled in a multicentre, non-randomised, open-label Phase II study (KEYNOTE-158), including patients with endometrial, gastric, small intestine, or biliary cancer. MSI or MMR tumour status was determined prospectively using PCR or IHC, respectively.
Patients received pembrolizumab 200 mg every 3 weeks until unacceptable toxicity or disease progression. Clinically stable patients with initial evidence of disease progression were permitted to remain on treatment until disease progression was confirmed. Patients without disease progression were treated for up to 24 months (up to 35 cycles). Assessment of tumour status was performed every 9 weeks through the first year, then every 12 weeks thereafter.
Among the 83 patients with endometrial cancer, the baseline characteristics were: median age of 64 years (range: 42 to 86), 46% age 65 or older; 84% White, 6% Asian, and 4% Black; and ECOG PS 0 (46%) and 1 (54%). Ninety-eight percent of the patients had M1 disease and 2% had M0 disease. Forty-seven percent of patients received 2 or more prior lines of therapy.
Among the 67 patients with gastric cancer, the baseline characteristics were: median age 68 years (range: 41 to 89); 61% age 65 or older; 64% male, 61% White, 25% Asian; and ECOG PS 0 (43%) and 1 (57%). All patients had M1 disease. Forty-five percent of patients received 2 or more prior lines of therapy.
Among the 33 patients with small intestinal cancer, the baseline characteristics were: median age 60 years (range: 21 to 78); 39% age 65 or older; 58% male, 85% White, 9% Asian; and ECOG PS 0 (52%) and 1 (48%). Ninety-seven percent of patients had M1 disease and 3% M0 disease. Thirty-three percent of patients received 2 or more prior lines of therapy. All patients had a tumour histology of adenocarcinoma.
Among the 22 patients with biliary cancer, the baseline characteristics were: median age 61 years (range: 40 to 77); 41% age 65 or older; 73% male, 91% White, 9% Asian; ECOG PS 0 (45%) and 1 (55%); and 82% M1 disease and 18% M0 disease. Forty-one percent of patients received 2 or more prior lines of therapy.
The primary efficacy outcome measure was ORR as assessed by BICR using RECIST 1.1. Secondary efficacy outcome measures included response duration, PFS, and OS. The median follow-up time in months was 53.5 (range: 1.5 to 99.4) for endometrial, 12.9 (range: 1.0 to 102.6) for gastric, 39.4 (range: 4.2 to 103.0) for small intestine, and 19.4 (range: 1.1 to 97.1) for biliary cancer. Efficacy results are summarised in Table 43.
Table 43. Efficacy results in KEYNOTE-158:
| Endpoint | Endometrial n=83 | Gastric¶ n=65 | Small Intestine¶ n=32 | Biliary n=22 |
|---|---|---|---|---|
| Objective response rate* | ||||
| ORR % (95% CI) | 52% (40.6, 62.9) | 40% (28.0, 52.9) | 63% (43.7, 78.9) | 45% (24.4, 67.8) |
| Complete response | 18% | 18% | 19% | 14% |
| Partial response | 34% | 22% | 44% | 32% |
| Response duration* | ||||
| Median in months (range) | NR (2.9, 91.9+) | NR (1.9+, 96.1+) | NR (3.7+, 91.4+) | NR (6.2, 92.1+) |
| % with duration ≥12 months# | 86% | 88% | 87% | 90% |
| % with duration ≥60 months# | 64% | 72% | 72% | 50% |
* Based on patients with a best objective response as confirmed complete or partial response
# Based on Kaplan-Meier estimation
+ Denotes there is no progressive disease by the time of last disease assessment
NR = not reached
¶ Efficacy analysis population consists of participants in the APaT population, who were enrolled at least 26 weeks prior to the cutoff date
Oesophageal carcinoma
KEYNOTE-590: Controlled study of combination therapy in oesophageal carcinoma patients naïve to treatment
The efficacy of pembrolizumab in combination with chemotherapy was investigated in KEYNOTE-590, a multicentre, randomised, double-blind, placebo-controlled study in patients with locally advanced unresectable or metastatic oesop hageal carcinoma or GEJ carcinoma (Siewert type I). Patients with active autoimmune disease, a medical condition that required immunosuppression, or known HER-2 positive GEJ adenocarcinoma patients were ineligible for the study. Randomisation was stratifie d by tumour histology (squamous cell carcinoma vs. adenocarcinoma), geographic region (Asia vs. ex-Asia), and ECOG performance status (0 vs. 1).
Patients were randomised (1:1) to one of the following treatment arms:
- Pembrolizumab 200 mg on Day 1 of each three-week cycle in combination with cisplatin 80 mg/m² IV on Day 1 of each three-week cycle for up to six cycles and 5-FU 800 mg/m² IV per day on Day 1 to Day 5 of each three -week cycle, or per local standard for 5-FU administration.
- Placebo on Day 1 of each three -week cycle in combination with cisplatin 80 mg/m² IV on Day 1 of each three -week cycle for up to six cycles and 5 -FU 800 mg/m² IV per day on Day 1 to Day 5 of each three -week cycle, or per local standard for 5-FU administration.
Treatment with pembrolizumab or chemotherapy continued until unacceptable toxicity or disease progression or a maximum of 24 months. Patients randomised to pembrolizumab were permitted to continue beyond the first RECIST v1.1-defined disease progression if clinically stable until the first radiographic evidence of disease progression was confirmed at least 4 weeks later with repeat imaging. Assessment of tumour status was performed every 9 weeks.
Among the 749 patients in KEYNOTE-590, 383 (51%) had tumours that expressed PD-L1 with a CPS ≥10 based on the PD-L1 IHC 22C3 pharmDxTM Kit. The baseline characteristics of these 383 patients were: median age of 63 years (range: 28 to 89), 41% age 65 or older; 82% male; 34% White and 56% Asian; 43% and 57% had an ECOG performance status of 0 and 1, respectively. Ninety-three percent had M1 disease. Seventy-five percent had a tumour histology of squamous cell carcinoma, and 25% had adenocarcinoma.
The primary efficacy outcome measures were OS and PFS as assessed by the investigator according to RECIST 1.1 in squamous cell histology, CPS ≥10, and in all patients. The study demonstrated a statistically significant improvement in OS and PFS for all pre-specified study populations. In all patients randomised to pembrolizumab in combination with chemotherapy, compared to chemotherapy the OS HR was 0.73 (95% CI 0.62-0.86) and the PFS HR was 0.65 (95% CI 0.55-0.76). Secondary efficacy outcome measures were ORR and duration of response, according to RECIST 1.1 as assessed by the investigator. Table 44 summarises key efficacy measures from the pre-specified analysis in patients whose tumours expressed PD-L1 with a CPS ≥10 in KEYNOTE-590 performed at a median follow-up time of 13.5 months (range: 0.5 to 32.7 months). The Kaplan-Meier curve for OS and PFS are shown in Figures 40 and 41.
Table 44. Efficacy results for pembrolizumab plus chemotherapy in KEYNOTE-590 with PD-L1 expression (CPS ≥10):
| Endpoint | Pembrolizumab Cisplatin Chemotherapy 5-FU n=186 | Standard Treatment* n=197 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 124 (66.7%) | 165 (83.8%) |
| Median in months† (95% CI) | 13.5 (11.1, 15.6) | 9.4 (8.0, 10.7) |
| Hazard ratio‡ (95% CI) | 0.62 (0.49, 0.78) | |
| p-Value§ | <0.0001 | |
| PFS¶ | ||
| Number (%) of patients with event | 140 (75.3%) | 174 (88.3%) |
| Median in months† (95% CI) | 7.5 (6.2, 8.2) | 5.5 (4.3, 6.0) |
| Hazard ratio‡ (95% CI) | 0.51 (0.41, 0.65) | |
| p-Value§ | <0.0001 | |
| Objective response rate¶ | ||
| ORR§ % (95% CI) | 51.1 (43.7, 58.5) | 26.9 (20.8, 33.7) |
| Complete response | 5.9% | 2.5% |
| Partial response | 45.2% | 24.4% |
| p-Value# | <0.0001 | |
| Response duration¶,Þ | ||
| Median in months (range) | 10.4 (1.9, 28.9+) | 5.6 (1.5+, 25.0+) |
| % with duration ≥6 months† | 80.2% | 47.7% |
| % with duration ≥12 months† | 43.7% | 23.2% |
| % with duration ≥18 months† | 33.4% | 10.4% |
* Cisplatin and 5-FU
† Based on Kaplan-Meier estimation
‡ Based on the stratified Cox proportional hazard model
§ One-sided p-Value based on log-rank test stratified by geographic region (Asia versus Rest of the World) and tumour histology (Adenocarcinoma versus Squamous Cell Carcinoma) and ECOG performance status (0 versus 1)
¶ Assessed by investigator using RECIST 1.1
# One-sided p-Value for testing. H0: difference in % = 0 versus H1: difference in % > 0
Þ Best objective response as confirmed complete response or partial response.
A total of 32 patients aged ≥75 years for PD-L1 CPS ≥10 were enrolled in KEYNOTE-590 (18 in the pembrolizumab combination and 14 in the control). Data about efficacy of pembrolizumab in combination with chemotherapy are too limited in this patient population.
Figure 40. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-590 with PD-L1 expression (CPS ≥10):
Figure 41. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-590 with PD-L1 expression (CPS ≥10):
Triple-negative breast cancer
KEYNOTE-522: Controlled study of neoadjuvant and adjuvant therapy in patients with locally advanced, inflammatory, or early-stage triple-negative breast cancer at high risk of recurrence
The efficacy of pembrolizumab in combination with chemotherapy as neoadjuvant treatment and then continued as monotherapy as adjuvant treatment after surgery was investigated in the randomised, double-blind, multicentre, placebo-controlled study KEYNOTE-522. If indicated, patients received adjuvant radiation therapy prior to or concurrent with adjuvant pembrolizumab or placebo. The key eligibility criteria for this study were locally advanced, inflammatory, or early-stage TNBC at high risk of recurrence (tumour size >1 cm but ≤2 cm in diameter with nodal involvement or tumour size >2 cm in diameter regardless of nodal involvement), regardless of tumour PD-L1 expression. Patients with active autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppre ssion were ineligible for the study. Randomisation was stratified by nodal status (positive vs. negative), tumour size (T1/T2 vs. T3/T4), and choice of carboplatin (dosed every 3 weeks vs. weekly). Patients were randomised (2:1) to receive either pembrolizumab or placebo via intravenous infusion:
- Four cycles of neoadjuvant pembrolizumab 200 mg every 3 weeks or placebo on Day 1 of cycles 1-4 of treatment regimen in combination with:
- Carboplatin
- AUC 5 mg/mL/min every 3 weeks on Day 1 of cycles 1-4 of treatment regimen or AUC 1.5 mg/mL/min every week on Day 1, 8, and 15 of cycles 1-4 of treatment regimen and
- Paclitaxel 80 mg/m² every week on Day 1, 8, and 15 of cycles 1-4 of treatment regimen
- Carboplatin
- Followed by four additional cycles of neoadjuvant pembrolizum ab 200 mg every 3 weeks or placebo on Day 1 of cycles 5-8 of treatment regimen in combination with:
- Doxorubicin 60 mg/m² or epirubicin 90 mg/m² every 3 weeks on Day 1 of cycles 5-8 of treatment regimen and
- Cyclophosphamide 600 mg/m² every 3 weeks on Day 1 of cycles 5-8 of treatment regimen
- Following surgery, 9 cycles of adjuvant pembrolizumab 200 mg every 3 weeks or placebo were administered.
Treatment with pembrolizumab or placebo continued until completion of the treatment (17 cycles), disease progression that precludes definitive surgery, disease recurrence in the adjuvant phase, or unacceptable toxicity.
A total of 1 174 patients were randomised. The study population characteristics were: median age of 49 years (range: 22 to 80); 11% age 65 or older; 99.9% female; 64% White; 20% Asian, 5% Black, and 2% American Indian or Alaska Native; ECOG performance status of 0 (87%) and 1 (13%); 56% were pre-menopausal status and 44% were post-menopausal status; 7% were primary Tumour 1 (T1), 68% T2, 19% T3, and 7% T4; 49% were nodal involvement 0 (N0), 40% N1, 11% N2, and 0.2% N3; 1.4% of patients had inflammatory breast cancer; 75% of patients were overall Stage II and 25% were Stage III.
The dual primary efficacy outcome measures were pCR rate and EFS. pCR was defined as absence of invasive cancer in the breast and lymph nodes (ypT0/Tis ypN0) and was assessed by the blinded local pathologist at the time of definitive surgery. EFS was defined as the time from randomisation to the first occurrence of any of the foll owing events: progression of disease that precludes definitive surgery, local or distant recurrence, second primary malignancy, or death due to any cause. A secondary efficacy outcome measure was OS.
The study demonstrated a statistically significant improvement in pCR rate difference at its pre-specified primary analysis (n=602), the pCR rates were 64.8% (95% CI: 59.9%, 69.5%) in the pembrolizumab arm and 51.2% (95% CI: 44.1%, 58 .3%) in the placebo arm, with a treatment difference of 13.6% (95% CI: 5.4%, 21.8%; p-Value 0.00055). The study also demonstrated a statistically significant improvement in EFS at its pre-specified interim analysis (median follow-up time for all patients of 37.8 months (range: 2.7-48.0 months), HR=0.63 (95% CI: 0.48, 0.82; p-Value 0.00031)). At a median follow -up time for all patients of 73.1 months (range: 2.7-83.9 months), the study also demonstrated a statistically significant improvement in OS.
Results reported from the pre -specified pCR final analysis (n=1002) and key efficacy measures from the EFS and OS pre-specified interim analysis at median follow-up time for all patients of 73.1 months (range: 2.7-83.9 months) are summarised in Table 45. The Kaplan-Meier curves for EFS and OS are shown in Figures 42 and 43.
Table 45. Efficacy results in KEYNOTE-522:
| Endpoint | Pembrolizumab with Chemotherapy/Pembrolizumab | Placebo with Chemotherapy/Placebo |
|---|---|---|
| pCR (ypT0/Tis ypN0)* | n=669 | n=333 |
| Number of patients with pCR | 428 | 182 |
| pCR Rate (%) (95% CI) | 64.0 (60.2, 67.6) | 54.7 (49.1, 60.1) |
| Treatment difference (%) estimate (95% CI)† | 9.2 (2.8, 15.6) | |
| p-Value‡ | 0.00221 | |
| EFS§ | n=784 | n=390 |
| Number (%) of patients with event | 123 (15.7%) | 93 (23.8%) |
| Hazard ratio (95% CI)¶ | 0.65 (0.51, 0.83) | |
| OSÞ | n=784 | n=390 |
| Number (%) of patients with event | 115 (14.7%) | 85 (21.8%) |
| Hazard ratio (95% CI)¶ | 0.66 (0.50, 0.87) | |
| p-Value# | 0.00150 | |
* Based on a pre-specified pCR final analysis (compared to a significance level of 0.0028)
† Based on Miettinen and Nurminen method stratified by nodal status, tumour size, and choice of carboplatin
‡ One-sided p- Value for testing. H0: difference in % = 0 versus H1: difference in % > 0
¶ Based on Cox regression model with Efron's method of tie handling with treatment as a covariate stratified by nodal status, tumour size, and choice of carboplatin
Þ Based on a pre-specified OS interim analysis (compared to a significance level of 0.00503)
# One-sided p-Value based on log-rank test stratified by nodal status, tumour size, and choice of carboplatin
Figure 42. Kaplan-Meier curve for event-free survival by treatment arm in KEYNOTE-522 (intent to treat population):
Figure 43. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-522 (intent to treat population):
KEYNOTE-355: Controlled study of combination therapy in TNBC patients previously untreated for metastatic disease
The efficacy of pembrolizumab in combination with paclitaxel, nab-paclitaxel, or gemcitabine and carboplatin was investigated in KEYNOTE-355, a randomised, double-blind, multicentre, placebo-controlled study. Key eligibility criteria were locally recurrent unresectable or metastatic TNBC, regardless of tumour PD-L1 expression, not previously treated with chemotherapy in the advanced setting. Patients with active autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomisation was stratified by chemotherapy treatment (paclitaxel or nab-paclitaxel vs. gemcitabine and carboplatin), tumour PD-L1 expression (CPS ≥1 vs. CPS <1), and prior treatment with the same class of chemotherapy in the neoadjuvant setting (yes vs. no). Patients were random ised (2:1) to one of the following treatment arms via intravenous infusion:
- Pembrolizumab 200 mg on Day 1 every 3 weeks in combination with nab-paclitaxel 100 mg/m² on Days 1, 8 and 15 every 28 days, or paclitaxel 90 mg/m² on Days 1, 8, and 15 every 28 days, or gemcitabine 1 000 mg/m² and carboplatin AUC 2 mg/mL/min on Days 1 and 8 every 21 days.
- Placebo on Day 1 every 3 weeks in combination with nab-paclitaxel 100 mg/m² on Days 1, 8 and 15 every 28 days, or paclitaxel 90 mg/m² on Days 1, 8, and 15 every 28 days, or gemcitabine 1 000 mg/m² and carboplatin AUC 2 mg/mL/min on Days 1 and 8 every 21 days.
Treatment with pembrolizumab or placebo, both in combination with chemotherapy, continued until RECIST 1.1-defined progression of disease as determined by the investigator, unacceptable toxicity, or a maximum of 24 months. Chemotherapy could continue per standard of care. Administration of pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and deriving clinical benefit as determined by the investigator. Assessment of tumour status was performed at Weeks 8, 16, and 24, then every 9 weeks for the first year, and every 12 weeks thereafter.
Among the 847 patients randomised in KEYNOTE-355, 636 (75%) had tumours that expressed PD-L1 with a CPS ≥1 and 323 (38%) had tumour PD-L1 expression CPS ≥10 based on the PD-L1 IHC 22C3 pharmDxTM Kit. The baseline characteristics of the 323 patients with tumour PD-L1 expression CPS ≥10 included: median age of 53 years (range: 22 to 83); 20% age 65 or older; 100% female; 69% White, 20% Asian, and 5% Black; ECOG performance status of 0 (61%) and 1 (39%); 67% were post-menopausal status; 3% had a history of brain metastases; and 20% had disease-free interval of <12 months.
The dual primary efficacy outcome measures were PFS as assessed by BICR using RECIST 1.1 and OS. Secondary efficacy outcome measures were ORR and response duration as assessed by BICR using RECIST 1.1. The study demonstrated a statistically significant imp rovement in PFS at its pre-specified interim analysis (HR 0.65; 95% CI 0.49, 0.86; p-Value 0.0012) and OS at final analysis for patients with tumour PD-L1 expression CPS ≥10 randomised to the pembrolizumab in combination with chemotherapy arm compared with placebo in combination with chemotherapy. Table 46 summarises key efficacy measures and Figures 44 and 45 show the Kaplan-Meier curves for PFS and OS based on the final analysis with a median follow-up time of 20.2 months (range: 0.3 to 53.1 months) for patients with tumour PD-L1 expression CPS ≥10.
Table 46. Efficacy results in KEYNOTE-355 patients with CPS ≥10:
| Endpoint | Pembrolizumab with chemotherapy* n=220 | Placebo with chemotherapy* n=103 |
|---|---|---|
| PFS† | ||
| Number (%) of patients with event | 144 (65%) | 81 (79%) |
| Hazard ratio‡ (95% CI) | 0.66 (0.50, 0.88) | |
| p-Value§ | 0.0018 | |
| Median in months (95% CI) | 9.7 (7.6, 11.3) | 5.6 (5.3, 7.5) |
| OS | ||
| Number (%) of patients with event | 155 (70%) | 84 (82%) |
| Hazard ratio‡ (95% CI) | 0.73 (0.55, 0.95) | |
| p-Value¶ | 0.0093 | |
| Median in months (95% CI) | 23.0 (19.0, 26.3) | 16.1 (12.6, 18.8) |
| Objective response rate† | ||
| ORR % (95% CI) | 53% (46, 59) | 41% (31, 51) |
| Complete response | 17% | 14% |
| Partial response | 35% | 27% |
| Response duration† | ||
| Median in months (range) | 12.8 (1.6+, 45.9+) | 7.3 (1.5, 46.6+) |
| % with duration ≥6 months# | 82% | 60% |
| % with duration ≥12 months# | 56% | 38% |
* Chemotherapy: paclitaxel, nab-paclitaxel, or gemcitabine and carboplatin.
† Assessed by BICR using RECIST 1.1.
‡ Based on Cox regression model with Efron's method of tie handling with treatment as a covariate stratified by chemotherapy on study (taxane vs. gemcitabine and carboplatin) and prior treatment with same class of chemotherapy in the neoadjuvant setting (yes vs. no).
§ Nominal p-Value based on log-rank test stratified by chemotherapy on study (taxane vs. gemcitabine and carboplatin) and prior treatment with same class of chemotherapy in the neoadjuvant setti ng (yes vs. no). At the pre-specified interim analysis of PFS (median follow-up time of 19.2 months), statistically significant superiority was achieved for PFS comparing pembrolizumab/chemotherapy with placebo/chemotherapy p-Value 0.0012.
¶ One-sided p-Value based on log-rank test stratified by chemotherapy on study (taxane vs. gemcitabine and carboplatin) and prior treatment with same class of chemotherapy in the neoadjuvant setting (yes vs. no). OS results met the pre-specified efficacy boundary of 0.011 3 for statistical significance.
# From product-limit (Kaplan-Meier) method for censored data.
+ Denotes there is no progressive disease by the time of last disease assessment.
Figure 44. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-355 patients with PD -L1 expression (CPS ≥10):
Figure 45. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-355 patients with PD-L1 expression (CPS ≥10):
Endometrial carcinoma
KEYNOTE-868 (NRG-GY018): Controlled study of combination therapy for treatment of patients with primary advanced or recurrent EC
The efficacy of pembrolizumab in combination with paclitaxel and carboplatin was investigated in KEYNOTE-868 (NRG-GY018), a randomised, multicentre, double-blind, placebo-controlled study in 810 patients with advanced or recurrent endometrial carcinoma including those with dMMR and pMMR tumours. Patients had not received prior systemic therapy or had received prior chemotherapy in the adjuvant setting. Patients who had received prior adjuvant chemotherapy were eligible if their chemotherapy-free interval was at least 12 months. Patients with endometrial sarcoma, including carcinosarcoma or patients with active autoimmune disease or a medical condition th at required immunosuppression were ineligible. Randomisation was stratified according to MMR status, ECOG PS (0 or 1 vs. 2), and prior adjuvant chemotherapy. Patients were randomised (1:1) to one of the following treatment arms:
- Pembrolizumab 200 mg every 3 weeks, paclitaxel 175 mg/m² and carboplatin AUC 5 mg/mL/min for 6 cycles, followed by pembrolizumab 400 mg every 6 weeks for up to 14 cycles.
- Placebo every 3 weeks, paclitaxel 175 mg/m² and carboplatin AUC 5 mg/mL/min for 6 cycles, followed by placebo every 6 weeks for up to 14 cycles.
All study medications were administered as an intravenous infusion on Day 1 of each treatment cycle. Treatment continued until disease progression, unacceptable toxicity or a maximum of 20 cycles (up to approximately 24 months). Patients with measurable disease who had RECIST-defined stable disease or partial response at the completion of cycle 6 were permitted to continue receiving paclitaxel and carboplatin with pembrolizumab or placebo for up to 10 cycles as determined by the investigator. Assessment of tumour status was performed every 9 weeks for the first 9 months and then every 12 weeks thereafter.
Among the 810 randomised patients, 222 (27%) had dMMR tumour status and 588 (73%) had pMMR tumour status.
The dMMR population characteristics were: median age of 66 years (range: 37 to 86), 55% age 65 or older; 79% White, 9% Black and 3% Asian; 5% Hispanic or Latino; 64% ECOG PS of 0, 33% ECOG PS of 1 and 3% ECOG PS of 2; 61% had recurrent disease and 39 % had primary or persistent disease; 5% received prior adjuvant chemotherapy and 43% received prior radiotherapy. The histologic subtypes were endometrioid carcinoma (24% grade 1, 43% grade 2, and 14% grade 3), adenocarcinoma Not Otherwise Specified (NOS) (11%), and other (8% including dedifferentiated/undifferentiated, serous and mixed epithelial).
The pMMR population characteristics were: median age of 66 years (range: 29 to 94), 54% age 65 or older; 72% White, 16% Black, and 5% Asian; 6% Hispanic or Latino; 67% ECOG PS of 0, 30% ECOG PS of 1 and 3% ECOG PS of 2; 56% had recurrent disease and 44% had primary or persistent disease; 26% received prior adjuvant chemotherapy and 41% received prior radiotherapy. The histologic subtypes were endometrioid carci noma (17% grade 1, 19% grade 2, and 16% grade 3), serous (26%), adenocarcinoma NOS (10%), clear cell carcinoma (7%), and other (5% including mixed epithelial and dedifferentiated/undifferentiated).
The primary efficacy outcome measure was PFS as assessed by the investigator according to RECIST 1.1 in the dMMR and pMMR populations. Secondary efficacy outcome measures included OS, ORR and response duration in the dMMR and pMMR populations. The study demonstrated statistically significant improvements in PFS for patients randomised to pembrolizumab in combination with chemotherapy compared to placebo in combination with chemotherapy in both the dMMR and pMMR populations. The median follow -up time was 13.6 months (range: 0.6 to 39.4 months) and 8.7 months (range: 0.1 to 37.2 months) in the dMMR and pMMR populations, respectively. OS endpoint was not formally assessed within multiplicity control. OS results were not mature. Efficacy results by MMR status are summarised in Table 47. The Kaplan-Meier curves for PFS by MMR status are shown in Figures 46 and 47, respectively.
Table 47. Efficacy results in KEYNOTE-868 (NRG-GY018):
| Endpoint | dMMR Population | pMMR Population | ||
|---|---|---|---|---|
| Pembrolizumab with chemotherapy* n=110 | Placebo with chemotherapy* n=112 | Pembrolizumab with chemotherapy* n=294 | Placebo with chemotherapy* n=294 | |
| PFS | ||||
| Number (%) of patients with event | 29 (26%) | 60 (54%) | 95 (32%) | 138 (47%) |
| Median in months (95% CI) | NR (30.7, NR) | 8.3 (6.5, 12.3) | 13.1 (10.6, 19.5) | 8.7 (8.4, 11.0) |
| Hazard ratio† (95% CI) | 0.34 (0.22, 0.53) | 0.57 (0.44, 0.74) | ||
| p-Value‡ | < 0.0001 | < 0.0001 | ||
| OS | ||||
| Number (%) of patients with event | 10 (9%) | 17 (15%) | 45 (15%) | 54 (18%) |
| Median in months (95% CI) | NR (NR, NR) | NR (NR, NR) | 28.0 (21.4, NR) | 27.4 (19.5, NR) |
| Hazard ratio† (95% CI) | 0.55 (0.25, 1.19) | 0.79 (0.53, 1.17) | ||
| Objective response rate | ||||
| Number of participants with measurable disease at baseline | n=95 | n=95 | n=220 | n=235 |
| ORR¶ % (95% CI) | 78% (68, 86) | 69% (59, 79) | 61% (55, 68) | 51% (45, 58) |
| Response duration | ||||
| Median in months (range) | NR (0.0+, 33.0+) | 4.4 (0.0+, 32.8+) | 7.1 (0.0+, 32.8+) | 6.4 (0.0+, 20.1+) |
* Chemotherapy (paclitaxel and carboplatin)
† Based on the stratified Cox proportional hazard model
‡ One-sided p-value based on stratified log -rank test (compared to an alpha boundary of 0.00207 for dMMR and 0.00116 for pMMR)
¶ Response: Best objective response as confirmed complete response or partial response
NR = not reached
Figure 46. Kaplan-Meier curve for progression-free survival in KEYNOTE-868 (NRG-GY018) in dMMR population:
Figure 47. Kaplan-Meier curve for progression-free survival in KEYNOTE-868 (NRG-GY018) in pMMR population:
KEYNOTE-775: Controlled study of combination therapy in advanced EC patients previously treated with systemic chemotherapy
The efficacy of pembrolizumab in combination with lenvatinib was investigated in KEYNOTE-775, a randomised, multicentre, open-label, active-controlled study conducted in patients with advanced EC who had been previously treated with at least one prior platinum -based chemotherapy regimen in any setting, including in the neoadjuvant and adjuvant settings. Participants may have received up to 2 platinum-containing therapies in total, as long as one was given in the neoadjuvant or adjuvant treatment setting. The study excluded patients with endometrial sarcoma, carcinosarcoma, pre-existing Grade ≥3 fistula, uncontrolled BP (>150/90 mmHg), significant cardiovascular impairment or event within previous 12 months, or patients who had active autoimmune disease or a medical condition that required immunosuppression. Randomisation was stratified by MMR status (dMMR or pMMR [mismatch repair proficient]) using a validated IHC test. The pMMR stratum was further stratified by ECOG performance status, geographic region, and history of pelvic radiation. Patients were randomised (1:1) to one of the following treatment arms:
- pembrolizumab 200 mg intravenously every 3 weeks in combination with lenvatinib 20 mg orally once dai ly.
- investigator's choice consisting of either doxorubicin 60 mg/m² every 3 weeks, or paclitaxel 80 mg/m² weekly, 3 weeks on/1 week off.
Treatment with pembrolizumab and lenvatinib continued until RECIST v1.1-defined progression of disease as verified by BICR, unacceptable toxicity, or for pembrolizumab, a maximum of 24 months. Administration of study treatment was permitted beyond RECIST -defined disease progression if the treating investigator considered the patient to be deriving clinical benefit and the treatment was tolerated. A total of 121/411 (29%) of the pembrolizumab and lenvatinib-treated patients received continued study therapy beyond RECIST-defined disease progression. The median duration of the post-progression therapy was 2.8 months. Assessment of tumour status was performed every 8 weeks.
A total of 827 patients were enrolled and randomised to pembrolizumab in combination with lenvatinib (n=411) or investigator's choice of doxorubicin (n=306) or paclitaxel (n=110). The baseline characteristics of these patients were: median age of 65 years (range: 30 to 86), 50% age 65 or older; 61% White, 21% Asian, and 4% Black; ECOG PS of 0 (59%) or 1 (41%), and 84% with pMMR tumour status and 16% with dMMR tumour status. The histologic subtyp es were endometrioid carcinoma (60%), serous (26%), clear cell carcinoma (6%), mixed (5%), and other (3%). All 827 of these patients received prior systemic therapy for EC: 69% had one, 28% had two, and 3% had three or more prior systemic therapies. 37% of patients received only prior neoadjuvant or adjuvant therapy.
The primary efficacy outcome measures were OS and PFS (as assessed by BICR using RECIST 1.1). Secondary efficacy outcome measures included ORR, as assessed by BICR using RECIST 1.1. At the pre-specified interim analysis, with a median follow-up time of 11.4 months (range: 0.3 to 26.9 months), the study demonstrated a statistically significant improvement in OS and PFS. The pre-specified final OS analysis with approximately 16 months of additional follow-up duration from the interim analysis (overall median follow-up time of 14.7 months [range: 0.3 to 43.0 months]) was performed without multiplicity adjustment. Efficacy results by MMR subgroups were consistent with overall study results. PFS, ORR and response duration results at the interim analysis and OS results at final analysis are summarised in Table 48. Kaplan-Meier curves for final OS and interim PFS analyses are shown in Figures 48 and 49, respectively.
Table 48. Efficacy results in KEYNOTE-775:
| Endpoint | Pembrolizumab 200 mg every 3 weeks Lenvatinib n=411 | Chemotherapy* n=416 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 276 (67%) | 329 (79%) |
| Median in months (95% CI) | 18.7 (15.6, 21.3) | 11.9 (10.7, 13.3) |
| Hazard ratio† (95% CI) | 0.65 (0.55, 0.77) | |
| p-ValueÞ | <0.0001 | |
| PFSß | ||
| Number (%) of patients with event | 281 (68%) | 286 (69%) |
| Median in months (95% CI) | 7.2 (5.7, 7.6) | 3.8 (3.6, 4.2) |
| Hazard ratio† (95% CI) | 0.56 (0.47, 0.66) | |
| p-Value‡ | <0.0001 | |
| Objective response rateß | ||
| ORR§ % (95% CI) | 32% (27, 37) | 15% (11, 18) |
| Complete response | 7% | 3% |
| Partial response | 25% | 12% |
| p-Value¶ | <0.0001 | |
| Response durationß | ||
| Median in months# (range) | 14.4 (1.6+, 23.7+) | 5.7 (0.0+, 24.2+) |
* Doxorubicin or Paclitaxel.
† Based on the stratified Cox regression model.
Þ One-sided nominal p-Value for final analysis based on stratified log-rank test. At the pre-specified interim analysis of OS with a median follow-up time of 11.4 months (range: 0.3 to 26.9 months), statistically significant superiority was achieved for OS comparing the combination of pembrolizumab and lenvatinib with chemotherapy (HR: 0.62 [95% CI: 0.51, 0.75] p-Value<0.0001)
ß At pre-specified interim analysis
‡ One-sided p-Value based on stratified log-rank test
§ Response: Best objective response as confirmed complete response or partial response
¶ Based on Miettinen and Nurminen method stratified by MMR Status, ECOG performance status, geographic region, and history of pelvic radiation
# Based on Kaplan-Meier estimation
Figure 48. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-775 (intent to treat population):
Figure 49. Kaplan-Meier curve for progression free-survival by treatment arm in KEYNOTE-775 (intent to treat population):
Cervical cancer
KEYNOTE-A18: Controlled study of combination therapy with CRT in patients with locally advanced cervical cancer
The efficacy of pembrolizumab in combination with cisplatin and external beam radiation therapy (EBRT) followed by brachytherapy (BT) was investigated in KEYNOTE-A18, a multicentre, randomised, double-blind, placebo-controlled study that enrolled 1 060 patients with locally advanced cervical cancer who had not previously received any definitive surgery, radiation, or systemic therapy for cervical cancer. There were 601 patients with FIGO (International Federation of Gynaecology and Obstetrics) 2014 Stage III - IVA (tumour involvement of the lower vagina with or without extension onto pelvic sidewall or hydronephrosis/non-functioning kidney or has spread to adjacent pelvic organs) with either node -positive or node -negative disease and 459 patients with FIGO 2014 Stage IB2 - IIB (tumour lesions >4 cm or clinically visible lesions that have spread beyond the uterus but have not extended onto the pelvic wall or to the lower third of vagina) with node -positive disease.
Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomisation was stratified by planned type of EBRT (Intensity-modulated radiation therapy [IMRT] or volumetric modulated arc therapy [VMAT] vs. non-IMRT and non-VMAT), stage at screening of cervical cancer (FIGO 2014 Stage IB2 - IIB vs. Stage III - IVA) and planned total radiotherapy dose ([EBRT + BT dose] of <70 Gy vs. ≥70 Gy as per equivalent dose [EQD2]). Patients were randomised (1:1) to one of the two treatment arms:
- Pembrolizumab 200 mg IV every 3 weeks (5 cycles) concurrent with cisplatin 40 mg/m² IV weekly (5 cycles, an optional sixth infusion could be administered per local practice) and radiotherapy (EBRT followed by BT), followed by pembrolizumab 400 mg IV every 6 weeks (15 cycles).
- Placebo IV every 3 weeks (5 cycles) concurrent with cisplatin 40 mg/m² IV weekly (5 cycles, an optional sixth infusion could be administered per local practice) and radiotherapy (EBRT followed by BT), followed by placebo IV every 6 weeks (15 cycles).
Treatment continued until RECIST v1.1-defined progression of disease as determined by the investigator or unacceptable toxicity. Assessment of tumo ur status was performed every 12 weeks for the first two years, every 24 weeks in year 3, and then annually.
Among the 601 patients with FIGO 2014 Stage III - IVA disease enrolled in KEYNOTE-A18, the baseline characteristics were: median age of 51 years (range: 22 to 87), 16% age 65 or older; 36% White, 1% Black, 34% Asian, 38% Hispanic or Latino; 68% ECOG performance status of 0 and 32% ECOG performance status of 1; 93% with CPS ≥1; 71% had positive pelvic and/or positive para-aortic lymph node (s), 29% had neither positive pelvic nor para-aortic lymph node, 86% IMRT or VMAT EBRT, 90% ≥70 Gy (EQD2). Eighty-four percent had squamous cell carcinoma and 16% had non-squamous histology.
The primary efficacy outcomes were PFS (as assessed by investigator according to RECIST v1.1 or histopathologic confirmation) and OS. The study demonstrated statistically significant improvements in PFS [0.70 (95% CI 0.55, 0.89; p-Value 0.0020)] from the first pre-specified interim analysis and OS [0.67 (95% CI 0.50, 0.90; p-Value 0.0040)] from the second pre-specified interim analysis in the overall population for patients randomised to pembrolizumab with CRT compared to placebo with CRT. Table 48 summarises key efficacy measures from the second pre-specified interim analysis in patients with FIGO 2014 Stage III - IVA disease with median follow-up time of 26.6 months (range: 0.9 to 41.7 months). The Kaplan-Meier curves in patients with FIGO 2014 Stage III - IVA disease for OS and PFS based on this analysis are shown in Figures 50 and 51, respectively.
Table 49. Efficacy results in KEYNOTE-A18 for patients with FIGO 2014 Stage III - IVA cervical cancer:
| Endpoint | Pembrolizumab 200 mg every 3 weeks and 400 mg every 6 weeks with CRT n=296 | Placebo with CRT n=305 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 43 (15%) | 73 (24%) |
| Median in months (95% CI) | NR (NR, NR) | NR (NR, NR) |
| Hazard ratio* (95% CI) | 0.57 (0.39, 0.83) | |
| PFS by investigator | ||
| Number (%) of patients with event | 79 (27%) | 125 (41%) |
| Median in months (95% CI) | NR (NR, NR) | NR (26.3, NR) |
| Hazard ratio* (95% CI) | 0.57 (0.43, 0.76) | |
* Based on the unstratified Cox proportional hazard model
CRT = Chemoradiotherapy
NR = not reached
Figure 50. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-A18 for patients with FIGO 2014 Stage III - IVA cervical cancer:
Figure 51. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-A18 for patients with FIGO 2014 Stage III - IVA cervical cancer:
KEYNOTE-826: Controlled study of combination therapy in patients with persistent, recurrent, or metastatic cervical cancer
The efficacy of pembrolizumab in combination with paclitaxel and cisplatin or paclitaxel and carboplatin, with or without bevacizumab, was investigated in KEYNOTE-826, a multicentre, randomised, double-blind, placebo-controlled study that enrolled 617 patients with persistent, recurrent, or first-line metastatic cervical cancer who had not been treated with chemotherapy except when used concurrently as a radio-sensitising agent. Patients were enrolled re gardless of tumour PD-L1 expression status. Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomisation was stratified by metastatic status at initial diagnosis, investigator decision to use bevacizumab, and PD-L1 status (CPS <1 vs. CPS 1 to <10 vs. CPS ≥10). Patients were randomised (1:1) to one of the two treatment groups:
- Treatment Group 1: Pembrolizumab 200 mg plus chemotherapy with or without bevacizumab
- Treatment Group 2: Placebo plus chemotherapy with or without bevacizumab
The investigator selected one of the following four treatment regimens prior to randomisation:
1. Paclitaxel 175 mg/m² + cisplatin 50 mg/m²
2. Paclitaxel 175 mg/m² + cisplatin 50 mg/m² + bevacizumab 15 mg/kg
3. Paclitaxel 175 mg/m² + carboplatin AUC 5 mg/mL/min
4. Paclitaxel 175 mg/m² + carboplatin AUC 5 mg/mL/min + bevacizumab 15 mg/kg
All study medications were administered as an intravenous infusion. All study treatments were administered on Day 1 of each 3-week treatment cycle. Cisplatin could be administered on Day 2 of each 3-week treatment cycle. The option to use bevacizumab was by investigator choice prior to randomisation. Treatment with pembrolizumab continued until RECIST v1.1-defined progression of disease, unacceptable toxicity, or a maximum of 24 months. Administration of pembrolizumab was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator. Assessment of tumo ur status was performed at Week 9 and then every 9 weeks for the first year, followed by every 12 weeks thereafter.
Of the 617 enrolled patients, 548 patients (89%) had tumours expressing PD-L1with a CPS ≥1 based on the PD-L1 IHC 22C3 pharmDxTM Kit. Among these 548 enrolled patients with tumours expressing PD-L1, 273 patients were randomised to pembrolizumab in combination with chemotherapy with or without bevacizumab, and 275 patients were randomised to placebo in combination with chemotherapy with or without bevacizumab. The baseline characteristics of these 548 patients were: median age of 51 years (range: 22 to 82), 16% age 65 or older; 59% White, 18% Asian, and 1% Black; 37% Hispanic or Latino; 56% and 43% ECOG performance status of 0 or 1, respectively; 63% received bevacizumab as study treatment; 21% with adenocarcinoma and 5% with adenosquamous histology; for patients with persistent or recurrent disease with or without distant metastases, 39% had received prior chemoradiation only and 17% had received prior chemoradiation plus surgery.
The primary efficacy outcome measures were OS and PFS as assessed by investigator according to RECIST v1.1. Secondary efficacy outcome measures were ORR and duration of response, according to RECIST v1.1, as assessed by investigator. At a pre-specified interim analysis, the study demonstrated statistically significant improvements in OS (HR 0.64; 95% CI 0.50, 0.81; p-Value = 0.0001) and PFS (HR 0.62; 95% CI 0.50, 0.77; p-Value < 0.0001) for patients whose tumours expressed PD-L1 with a CPS ≥1 randomised to pembrolizumab in combination with chemotherapy with or without bevacizumab compared to placebo in combination with chemotherapy with or without bevacizumab. The study also demonstrated statistically significant improvements in OS and PFS in the overall population. Table 50 summarises key efficacy measures for patients whose tumours expressed PD-L1 with a CPS ≥1 in KEYNOTE-826 at the final analysis with a median duration of follow-up of 21.3 months. The Kaplan-Meier curves for OS and PFS based on the final analysis are shown in Figures 52 and 53.
Table 50. Efficacy results in KEYNOTE-826 for patients with PD-L1 expression (CPS ≥1):
| Endpoint | Pembrolizumab 200 mg every 3 weeks plus Chemotherapy* with or without bevacizumab n=273 | Placebo plus Chemotherapy* with or without bevacizumab n=275 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 153 (56%) | 201 (73%) |
| Median in months (95% CI) | 28.6 (22.1, 38.0) | 16.5 (14.5, 20.0) |
| Hazard ratio† (95% CI) | 0.60 (0.49, 0.74) | |
| p-Value‡ | <0.0001 | |
| PFS | ||
| Number (%) of patients with event | 171 (63%) | 220 (80%) |
| Median in months (95% CI) | 10.5 (9.7, 12.3) | 8.2 (6.3, 8.5) |
| Hazard ratio† (95% CI) | 0.58 (0.47, 0.71) | |
| p-Value‡ | <0.0001 | |
| Objective response rate | ||
| ORR¶ % (95% CI) | 69% (63, 74) | 51% (45, 57) |
| Complete response | 26% | 15% |
| Partial response | 43% | 36% |
| Response duration | ||
| Median in months (range) | 19.2 (1.3+, 40.9+) | 10.4 (1.5+, 40.7+) |
| % with duration ≥12 months# | 56 | 45 |
| % with duration ≥24 months# | 48 | 30 |
* Chemotherapy (paclitaxel and cisplatin or paclitaxel and carboplatin).
† Based on the stratified Cox proportional hazard model.
‡ Nominal p-Value based on stratified log-rank test.
¶ Response: Best objective response as confirmed complete response or partial response.
# Based on Kaplan-Meier estimation.
Figure 52. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-826 patients with PD-L1 expression (CPS ≥1):
* Chemotherapy (paclitaxel and cisplatin or paclitaxel and carboplatin) with or without bevacizumab
Figure 53. Kaplan-Meier curve for progression free survival by treatment arm in KEYNOTE-826 patients with PD -L1 expression (CPS ≥1):
* Chemotherapy (paclitaxel and cisplatin or paclitaxel and carboplatin) with or without bevacizumab.
Gastric or gastro-oesophageal junction (GEJ) adenocarcinoma
KEYNOTE-811: Controlled study of combination therapy in locally advanced unresectable or metastatic HER2-positive gastric or gastro-oesophageal junction adenocarcinoma patients naïve to treatment
The efficacy of pembrolizumab in combination with trastuzumab plus fluoropyrimidine and platinum-containing chemotherapy was investigated in KEYNOTE-811, a multicentre, randomised, double-blind, placebo-controlled study that enrolled 698 patients with HER2-positive advanced gastric or GEJ adenocarcinoma regardless of PD-L1 expression status, who had not previously received systemic therapy for metastatic disease. Patients with an autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible.
Randomisation was stratified by PD-L1 expression (CPS ≥1 or <1), chemotherapy regimen (5-FU plus cisplatin [FP] or capecitabine plus oxaliplatin [CAPOX]), and geographic region (Europe/Israel/North America/Australia, Asia or Rest of the World). Patients were randomised (1:1) to one of the following treatment arms; all study medications, except oral capecitabine, were administered as an intravenous infusion for every 3-week treatment cycle:
- Pembrolizumab 200 mg, trastuzumab 8 mg/kg on first infusion and 6 mg/kg in subsequent cycles, followed by investigator's choice of combination chemotherapy of cisplatin 80 mg/m² for up to 6 cycles and 5-FU 800 mg/m²/day for 5 days (FP) or oxaliplatin 130 mg/m² up to 6-8 cycles and capecitabine 1 000 mg/m² bid for 14 days (CAPOX). Pembrolizumab was administered prior to trastuzumab and chemotherapy on Day 1 of each cycle.
- Placebo, trastuzumab 8 mg/kg on first infusion and 6 mg/kg in subsequent cycles, followed by investigator's choice of combination chemotherapy of cisplatin 80 mg/m² for up to 6 cycles and 5-FU 800 mg/m²/day for 5 days (FP) or oxaliplatin 130 mg/m² up to 6-8 cycles and capecitabine 1 000 mg/m² bid for 14 days (CAPOX). Placebo was administered prior to trastuzumab and chemotherapy on Day 1 of each cycle.
Treatment with pembrolizumab, trastuzumab and chemotherapy or placebo, trastuzumab and chemotherapy continued until RECIST v1.1-defined progression of disease as determined by BICR, unacceptable toxicity, or a maximum of 24 months. Assessment of tumo ur status was performed every 6 weeks.
Among the 698 patients randomised in KEYNOTE-811, 594 (85%) had tumours that expressed PD-L1 with a CPS ≥1 based on the PD-L1 IHC 22C3 pharmDxTM kit. The baseline characteristics of the 594 patients with tumour PD-L1 expression CPS ≥1 included: median age of 63 years (range: 19 to 85), 43% age 65 or older; 80% male; 63% White, 33% Asian, and 0.7% Black; 42% ECOG PS of 0 and 58% ECOG PS of 1. Ninety-eight percent of patients had metastatic disease (stage IV) and 2% had locally advanced unresectable disease. Ninety-five percent (n=562) had tumours that were not MSI H, 1% (n=8) had tumours that were MSI H, and in 4% (n=24) the status was not known. Eighty-five percent of patients received CAPOX.
The primary efficacy outcome measures were PFS based on BICR using RECIST 1.1, and OS. Secondary efficacy outcome measures included ORR and DoR based on BICR using REC IST 1.1. At the second interim analysis in the overall population, the study demonstrated a statistically significant improvement in PFS (HR 0.72; 95% CI 0.60, 0.87; p-Value 0.0002) for patients randomised to the pembrolizumab arm in combination with tras tuzumab and chemotherapy compared with placebo in combination with trastuzumab and chemotherapy. At this interim analysis, there was no statistically significant difference with respect to OS. The median follow-up time was 15.4 months (range: 0.3 to 41.6 months). At the first interim analysis conducted on the first 264 patients randomised in the overall population (133 patients in the pembrolizumab arm and 131 in the placebo arm), a statistically significant improvement was observed in ORR (74.4% vs. 51.9%, representing a 22.7% difference in ORR, [95% CI: 11.2, 33.7]; p-Value 0.00006).
Table 51 summarises key efficacy results at the second interim analysis for the pre-specified subgroup of patients whose tumours expressed PD-L1 with a CPS ≥1 and Figures 54 and 55 show the Kaplan-Meier curves for PFS and OS
Table 51. Efficacy results for KEYNOTE-811 for patients with PD-L1 expression (CPS ≥1):
| Endpoint | Pembrolizumab Trastuzumab and Chemotherapy n=298 | Placebo Trastuzumab and Chemotherapy n=296 |
|---|---|---|
| PFS | ||
| Number (%) of patients with event | 199 (67%) | 215 (73%) |
| Median in months (95% CI) | 10.8 (8.5, 12.5) | 7.2 (6.8, 8.4) |
| Hazard ratio* (95% CI) | 0.7 (0.58, 0.85) | |
| p-Value† | 0.0001 | |
| OS | ||
| Number (%) of patients with event | 167 (56%) | 183 (62%) |
| Median in months (95% CI) | 20.5 (18.2, 24.3) | 15.6 (13.5, 18.6) |
| Hazard ratio* (95% CI) | 0.79 (0.64, 0.98) | |
| p-Value† | 0.0143 | |
| Objective response rate | ||
| ORR‡ % (95% CI) | 73% (67.7, 78.1) | 58% (52.6, 64.1) |
| Complete response | 14% | 10% |
| Partial response | 59% | 49% |
| p-Value# | 0.00008 | |
| Response duration | ||
| Median in months (range) | 11.3 (1.1+, 40.1+) | 9.5 (1.4+, 38.3+) |
| % with duration ≥6 months¶ | 75% | 67% |
| % with duration ≥12 months¶ | 49% | 41% |
* Based on unstratified Cox proportional hazard model
† Nominal p-value based on unstratified log-rank test; no formal test was performed in patients with PD-L1 expression (CPS ≥1).
‡ Response: Best objective response as confirmed complete response or partial response
# Nominal p-value based on unstratified Miettinen and Nurminen method; no formal test was performed in patients with PD-L1 expression (CPS ≥1).
¶ Based on Kaplan-Meier estimation
Figure 54. Kaplan-Meier curve for progression free survival by treatment arm in KEYNOTE-811 patients with PD-L1 expression (CPS ≥1):
Figure 55. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-811 patients with PD-L1 expression (CPS ≥1):
KEYNOTE-859: Controlled study of combination therapy in locally advanced unresectable or metastatic HER2-negative gastric or gastro-oesophageal junction adenocarcinoma patients naïve to treatment
The efficacy of pembrolizumab in combination with fluoropyrimidine and platinum chemotherapy was investigated in KEYNOTE-859, a multicentre, randomised, double-blind, placebo-controlled study that enrolled 1 579 patients with HER2-negative advance d gastric or GEJ adenocarcinoma regardless of PD-L1 expression status, who had not previously received systemic therapy for metastatic disease. Prior neoadjuvant and/or adjuvant therapy was allowed if it was completed at least 6 months prior to randomisation. Patients with an autoimmune disease that required systemic therapy within 2 years of treatment, a medical condition that required immunosuppression, or patients who had received prior treatment with immune checkpoint inhibitors were ineligible.
Randomisation was stratified by PD-L1 expression (CPS ≥1 or <1), chemotherapy regimen (5-FU plus cisplatin [FP] or capecitabine plus oxaliplatin [CAPOX]), and geographic region (Europe/Israel/North America/Australia, Asia or Rest of the World).
Patients were randomised (1:1) to one of the following treatment arms; all study medications, except oral capecitabine, were administered as an intravenous infusion for every 3-week treatment cycle:
- Pembrolizumab 200 mg, investigator's choice of combination chemotherapy of cisplatin 80 mg/m² and 5-FU 800 mg/m²/day for 5 days (FP) or oxaliplatin 130 mg/m² and capecitabine 1 000 mg/m² bid for 14 days (CAPOX) for up to 35 cycles. Duration of cisplatin or oxaliplatin treatment could be capped at 6 cycles as per local country guidelines. Pembrolizumab was administered prior to chemotherapy on Day 1 of each cycle.
- Placebo, investigator's choice of combination chemotherapy of cisplatin 80 mg/m² and 5-FU 800 mg/m²/day for 5 days (FP) or oxaliplatin 130 mg/m² and capecitabine 1 000 mg/m² bid for 14 days (CAPOX) for up to 35 cycles. Duration of cisplatin or oxaliplatin treatment could be capped at 6 cycles as per local country guidelines. Placebo was administered prior to chemotherapy on Day 1 of each cycle.
Treatment with pembrolizumab and chemotherapy or placebo and chemotherapy continued until RECIST v1.1-defined progression of disease as determined by BICR, unacceptable toxicity, or a maximum of 24 months. Assessment of tumour status was performed every 6 weeks.
Among the 1 579 patients in KEYNOTE-859, 1 235 (78%) had tumours that expressed PD-L1 with a CPS ≥1 based on the PD-L1 IHC 22C3 pharmDxTM kit. The baseline characteristics of the 1 235 patients with tumour PD-L1 expression CPS ≥1 included: median age of 62 years (range: 24 to 86), 40% age 65 or older; 70.4% male; 55.5% White; 33.1% Asian; 36.5% ECOG PS of 0 and 63.5% ECOG PS of 1. Ninety-six percent of patients had metastatic disease (stage IV) and 4% had locally advanced unresectable disease. Five percent (n=66) had tumours that were MSI-H. Eighty-six percent of patients received CAPOX.
The primary efficacy outcome measure was OS. Additional secondary efficacy outcome measures included PFS, ORR, and DOR as assessed by BICR using RECIST 1.1.
The study demonstrated a statistically significant improvement in OS (HR 0.78; 95% CI 0.70, 0.87; p-Value <0.0001), PFS (HR 0.76; 95% CI 0.67, 0.85; p-Value <0.0001) and ORR (51% [95% CI 47.7, 54.8] vs 42% [95% CI 38.5, 45.5]; p-Value 0.00009) in patients randomised to pembrolizumab in combination with chemotherapy compared with placebo in combination with chemotherapy in the overall population. The median follow-up time was 12 months (range: 0.1 to 45.9 months). Table 52 summarises key efficacy results for the pre-specified subgroup of patients whose tumours expressed PD-L1 with a CPS ≥1 and Figures 56 and 57 show the Kaplan-Meier curves for OS and PFS.
Table 52. Efficacy results in KEYNOTE-859 for patients with PD L1 expression (CPS ≥1):
| Endpoint | Pembrolizumab Fluoropyrimidine and Platinum Chemotherapy n=618 | Placebo Fluoropyrimidine and Platinum Chemotherapy n=617 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 464 (75%) | 526 (85%) |
| Median in months* (95% CI) | 13.0 (11.6, 14.2) | 11.4 (10.5, 12.0) |
| Hazard ratio† (95% CI) | 0.74 (0.65, 0.84) | |
| p-Value‡ | <0.0001 | |
| PFS | ||
| Number (%) of patients with event | 443 (72%) | 483 (78%) |
| Median in months* (95% CI) | 6.9 (6.0, 7.2) | 5.6 (5.4, 5.7) |
| Hazard ratio† (95% CI) | 0.72 (0.63, 0.82) | |
| p-Value‡ | <0.0001 | |
| Objective response rate | ||
| ORR§ (95% CI) | 52% (48.1, 56.1) | 43% (38.7, 46.6) |
| Complete response | 10% | 6% |
| Partial response | 42% | 37% |
| p-Value¶ | 0.00041 | |
| Response duration | ||
| Median in months* (range) | 8.3 (1.2+, 41.5+) | 5.6 (1.3+, 34.2+) |
| % with duration ≥12 months* | 41% | 26% |
* Based on Kaplan-Meier estimation
† Based on the stratified Cox proportional hazard model
‡ One sided p-Value based on stratified log-rank test
§ Response: Best objective response as confirmed complete response or partial response
¶ One sided p-Value based on stratified Miettinen and Nurminen method
Figure 56. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-859 patients with PD-L1 expression (CPS ≥1):
Figure 57. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-859 patients with PD-L1 expression (CPS ≥1):
An analysis was performed in KEYNOTE-859 in patients who se tumours expressed PD-L1 with a CPS ≥1 to <10 or CPS ≥10 in both arms (see Table 52).
Table 53. Efficacy results by PD-L1 expression in KEYNOTE-859:
| Endpoint | Pembrolizumab combination therapy n=337 | Chemotherapy n=345 | Pembrolizumab combination therapy n=279 | Chemotherapy n=272 |
|---|---|---|---|---|
| CPS ≥1 to <10 | CPS ≥10 | |||
| OS HR (95% CI) | 0.83 (0.70, 0.98)* | 0.65 (0.53, 0.79)† | ||
| PFS HR (95% CI) | 0.83 (0.70, 0.99)* | 0.62 (0.51, 0.76)† | ||
| ORR§ (95% CI) | 45% (39.7, 50.6) | 42% (37.0, 47.7) | 61% (54.6, 66.3) | 43% (37.1, 49.1) |
* Hazard ratio (pembrolizumab combination therapy compared to chemotherapy) based on unstratified Cox proportional hazard model
† Hazard ratio (pembrolizumab combination therapy compared to chemotherapy) based on stratified Cox proportional hazard model
§ Response: Best objective response as confirmed complete response or partial response
Biliary tract carcinoma
KEYNOTE-966: Controlled study of combination therapy in patients with locally advanced unresectable or metastatic BTC
The efficacy of pembrolizumab in combination with gemcitabine and cisplatin was investigated in KEYNOTE-966, a multicentre, randomised, double-blind, placebo-controlled study that enrolled 1 069 patients with locally advanced unresectable or metastatic BTC, who had not received prior systemic therapy in the advanced disease setting. Patients were enrolled regardless of tumour PD-L1 expression. Patients must have had acceptable serum bilirubin levels (≤1.5 x ULN or direct bilirubin ≤ ULN for participants with total bilirubin levels >1.5 × ULN) and any clinically significant biliary obstruction had to be resolved before randomisation. Patients with autoimmune disease that required systemic therapy within 2 years of treatment or a medical condition that required immunosuppression were ineligible. Randomisation was stratified by geographic region (Asia vs. non-Asia), locally advanced versus metastatic, and site of origin (gallbladder, intrahepatic or extrahepatic cholangiocarcinoma).
Patients were randomised (1:1) to one of the two treatment groups:
- Pembrolizumab 200 mg on Day 1 plus gemcitabine 1 000 mg/m² and cisplatin 25 mg/m² on Day 1 and Day 8 every 3 weeks
- Placebo on Day 1 plus gemcitabine 1 000 mg/m² and cisplatin 25 mg/m² on Day 1 and Day 8 every 3 weeks
All study medications were administered via intravenous infusion. Treatment was continued until unacceptable toxicity or disease progression. For pembrolizumab, treatment was continued for a maximum of 35 cycles, or approximately 24 months. For cisplatin, treatment could be administered for a maximum of 8 cycles and for gemcitabine, treatment could be continued beyond 8 cycles. Assessment of tumo ur status was performed at baseline and then every 6 weeks through 54 weeks followed by every 12 weeks thereafter.
The study population characteristics were median age of 64 years (range: 23 to 85), 47% age 65 or older; 52% male; 49% White, 46% Asian; 46% ECOG PS of 0 and 54% ECOG PS of 1; 31% of patients had a history of hepatitis B infection, and 3% had a history of hepatitis C infection.
The primary efficacy outcome measure was OS and the secondary efficacy measures were PFS, ORR and DOR as assessed by BICR using RECIST 1.1. The study demonstrated a statistically significant improvement in OS at final analysis for patients randomised to pembrolizumab in combination with chemotherapy compared to placebo in combination with chemotherapy. Table 54 summarises key efficacy measures and Figures 58 and 59 show the Kaplan-Meier curves for PFS and OS based on the final analysis with a median follow -up time of 11.6 months (range: 0.2 to 37.5 months).
Table 54. Efficacy results in KEYNOTE-966:
| Endpoint | Pembrolizumab 200 mg every 3 weeks with gemcitabine/cisplatin n=533 | Placebo with gemcitabine/cisplatin n=536 |
|---|---|---|
| OS | ||
| Number (%) of patients with event | 414 (78%) | 443 (83%) |
| Median in months (95% CI) | 12.7 (11.5, 13.6) | 10.9 (9.9, 11.6) |
| Hazard ratio* (95% CI) | 0.83 (0.72, 0.95) | |
| p-Value† | 0.0034 | |
| PFS | ||
| Number (%) of patients with event | 428 (80%) | 448 (84%) |
| Median in months (95% CI) | 6.5 (5.7, 6.9) | 5.6 (4.9, 6.5) |
| Hazard ratio* (95% CI) | 0.87 (0.76, 0.99) | |
| p-Value‡ | 0.0171 | |
| Objective response rate | ||
| ORR% (95% CI) | 29.3% (25.4, 33.3) | 28.4% (24.6, 32.4) |
| Complete response | 2.6% | 1.7% |
| Partial response | 26.6% | 26.7% |
| p-Valueα | 0.3610 | |
| Response duration§,¶ | ||
| Median in months (range) | 8.3 (1.2+, 33.0+) | 6.8 (1.1+, 30.0+) |
| % with duration ≥6 months¶ | 65% | 55% |
| % with duration ≥12 months¶ | 38% | 27% |
* Based on the stratified Cox proportional hazard model
† One-sided p-Value based on a stratified log-rank test. The OS result met the pre-specified one-sided significance level of 0.0200
‡ One-sided p-Value based on stratified log-rank test. The PFS result did not meet the pre-specified one-sided significance level of 0.0125
α One-sided p-Value based on the stratified Miettinen and Nurminen method. The ORR result did not meet the pre-specified one-sided significance level of 0.0125
§ Based on patients with objective response that is confirmed complete response or partial response
¶ Based on Kaplan-Meier estimate
Figure 58. Kaplan-Meier curve for overall survival by treatment arm in KEYNOTE-966 (intent to treat population):
Figure 59. Kaplan-Meier curve for progression-free survival by treatment arm in KEYNOTE-966 (intent to treat population):
Elderly population
No overall differences in safety were observed in patients ≥75 years of age compared to younger patients receiving pembrolizumab monotherapy. Based on limited safety data from patients ≥75 years of age, when administrated in combination with chemotherapy, pembrolizumab showed less tolerability in patients ≥75 years of age compared to younger patients. For efficacy data in patients ≥75 years of age please refer to the relevant section of each indication.
Paediatric population
In KEYNOTE-051, 161 paediatric patients (62 children aged 9 months to less than 12 years and 99 adolescents aged 12 years to 17 years) with advanced melanoma or PD-L1 positive advanced, relapsed, or refractory solid tumours or lymphoma were administered pembrolizumab 2 mg/kg bw every 3 weeks. All patients received pembrolizumab for a median of 4 doses (range: 1-35 doses), with 138 patients (85.7%) receiving pembrolizumab for 2 doses or more. Participants were enrolled across 28 tumour types by primary diagnosis. The most common tumour types by histology were Hodgkin lymphoma (13.7%), glioblastoma multiforme (9.3%), neuroblastoma (6.2%), osteosarcoma (6.2%) and melanoma (5.6%). Of the 161 patients, 137 were enrolled with solid tumours, 22 with Hodgkin lymphoma, and 2 with other lymphomas. In patients with solid tumours and other lymphomas, the ORR was 5.8%, no patient had a complete response and 8 patients (5.8%) had a partial response. In the Hodgkin lymphoma population (n=22), in patients aged 11 years to 17 years, the baseline characteristics were median age 15 years; 64% male; 68% White; 77% had a Lansky/Karnofsky scale 90-100 and 23% had scale 70-80. Eighty-six percent had two or more prior lines of therapy and 64% 130 had Stage 3 or higher. In these paediatric patients with cHL, the ORR assessed by BICR according to the IWG 2007 criteria was 54.5%, 1 patient (4.5%) had a complete response and 11 patients (50.0%) had a partial response, and the ORR assessed by the Lugano 2014 criteria was 63.6%, 4 patients (18.2%) had a complete response and 10 patients (45.5%) had a partial response. Data from clinical trials in adolescent melanoma patients is very limited and extrapolation from adult data has been used to establish efficacy. Among the 5 adolescent participants with advanced melanoma treated on KEYNOTE-051, no patient had a complete or a partial response, and 1 patient had stable disease.
The European Medicines Agency has deferred the obligation to submit the results of studies with pembrolizumab in one or more subsets of the paediatric population in treatment of Hodgkin lymphoma (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
The pharmacokinetics of pembrolizumab was studied in 2 993 patients with metastatic or unresectable melanoma, NSCLC, or carcinoma who received doses in the range: of 1 to 10 mg/kg bw every 2 weeks, 2 to 10 mg/kg bw every 3 weeks, or 200 mg every 3 weeks.
Absorption
Pembrolizumab is administered via the intravenous route and therefore is immediately and completely bioavailable.
Distribution
Consistent with a limited extravascular distribution, the volume of distribution of pembrolizumab at steady-state is small (~6.0 L; CV: 20%). As expected for an antibody, pembrolizumab does not bind to plasma proteins in a specific manner.
Biotransformation
Pembrolizumab is catabolised through non-specific pathways; metabolism does not contribute to its clearance.
Elimination
Pembrolizumab CL is approximately 23% lower (geometric mean, 195 mL/day [CV%: 40%]) after achieving maximal change at steady-state compared with the first dose (252 mL/day [CV%: 37%]); this decrease in CL with time is not considered clinically meaningful. The geometric mean value (CV%) for the terminal half-life is 22 days (32%) at steady-state.
Linearity/non-linearity
Exposure to pembrolizumab as expressed by peak concentration (Cmax) or area under the plasma concentration time curve (AUC) increased dose proportionally within the dose range for efficacy. Steady-state concentrations of pembrolizumab were reached by 16 weeks of repeated dosing with an every 3 week regimen and the systemic accumulation was 2.1-fold. The median trough concentrations (Cmin) at steady-state were approximately 22 mcg/mL at a dose of 2 mg/kg bw every 3 weeks and 29 mcg/mL at a dose of 200 mg every 3 weeks. The median area under the concentration time curve at steady-state over 3 weeks (AUC0-3weeks) was 794 mcg•day/mL at a dose of 2 mg/kg bw every 3 weeks and 1 053 mcg•day/mL at a dose of 200 mg every 3 weeks.
Following administration of pembrolizumab 200 mg every 3 weeks in patients with cHL, the observed median Cmin at steady-state was up to 40% higher than that in other tumour types treated with the same dosage; however, the range of trough concentrations is similar. There are no notable differences in median Cmax between cHL and other tumour types. Based on available safety data in cHL and other tumour types, these differences are not clinically meaningful.
Special populations
The effects of various covariates on the pharmacokinetics of pembrolizumab were assessed in population pharmacokinetic analyses. The following factors had no clinically important effect on the clearance of pembrolizumab: age (range: 15-94 years), gender, race, mild or moderate renal impairment, mild or moderate hepatic impairment and tumour burden. The relationship between body weight and clearance supports the use of either fixed dose or body weight-based dosing to provide adequate and similar control of expos ure. Pembrolizumab exposure with weight-based dosing at 2 mg/kg bw every 3 weeks in paediatric patients (≥3 to 17 years) are comparable to those of adults at the same dose.
Renal impairment
The effect of renal impairment on the clearance of pembrolizumab was evaluated by population pharmacokinetic analyses in patients with mild or moderate renal impairment compared to patients with normal renal function. No clinically important differences in the clearance of pembrolizumab were found between patients with mild or moderate renal impairment and patients with normal renal function. Pembrolizumab has not been studied in patients with severe renal impairment (see section 4.2).
Hepatic impairment
The effect of hepatic impairment on the clearance of pembrolizumab was evaluated by population pharmacokinetic analyses in patients with mild and moderate hepatic impairment (as defined using the US National Cancer Institute criteria of hepatic dysfunction) compared to patients with normal hepatic function. No clinically important differences in the clearance of pembrolizumab were found between patients with mild or moderate hepatic impairment and normal hepatic function. Pembrolizumab has not been studied in patients with severe hepatic impairment (see section 4.2).
Preclinical safety data
The safety of pembrolizumab was evaluated in a 1-month and a 6-month repeat-dose toxicity study in Cynomolgus monkeys administered intravenous doses of 6, 40 or 200 mg/kg bw once a week in the 1-month study and once every two weeks in the 6-month study, followed by a 4-month treatment-free period. No findings of toxicological significance were observed and the no observed adverse effect level (NOAEL) in both studies was ≥200 mg/kg bw, which produced exposure multiples of 19 and 94 times the exposure in humans at doses of 10 and 2 mg/kg bw, respectively. The exposure multiple between the NOAEL and a human dose of 200 mg was 74.
Animal reproduction studies have not been conducted with pembrolizumab. The PD-1/PD-L1 pathway is thought to be involved in maintaining tolerance to the foetus throughout pregnancy. Blockade of PD-L1 signalling has been shown in murine models of pregnancy to disrupt tolerance to the foetus and to result in an increase in foetal loss.
Animal fertility studies have not been conducted with pembrolizumab. In 1 month and 6 month repeat-dose toxicology studies in monkeys, there were no notable effects in the male and female reproductive organs; however, many animals in these studies were not sexually mature.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.