KISUNLA Solution for injection Ref.[110679] Active ingredients: Donanemab
Source: FDA, National Drug Code (US) Revision Year: 2024
12. Clinical Pharmacology
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADAs in other studies, including those of donanemab-azbt or of other donanemab products.
In up to 18 months of treatment in Study 1, 87% (691/792) of patients receiving KISUNLA once monthly developed antidonanemab-azbt antibodies, and of those, 100% (691/691) had neutralizing antibodies.
Anti-donanemab-azbt antibody formation was associated with a higher incidence of infusion-related reactions compared to placebo [see Adverse Reactions (6.1)].
Anti-Drug Antibody Effects on Pharmacokinetics and Pharmacodynamics
The presence of anti-donanemab-azbt antibodies increased donanemab-azbt clearance. Among patients treated with KISUNLA in the placebo-controlled studies who developed anti-donanemab-azbt antibodies, mean donanemab-azbt serum trough concentrations at various time points were lower compared to patients who had not developed antidonanemab-azbt antibodies. Patients with high ADA titers showed less reduction in amyloid plaque compared to patients with low ADA titers. However, there was no identified clinically significant effect of anti-donanemab-azbt antibodies on the effectiveness of KISUNLA over the treatment duration of 18 months.
12.1. Mechanism of Action
Donanemab-azbt is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against insoluble N-truncated pyroglutamate amyloid beta. The accumulation of amyloid beta plaques in the brain is a defining pathophysiological feature of Alzheimer’s disease. Donanemab-azbt reduces amyloid beta plaques, as evaluated in Study 1 [see Clinical Studies (14)].
12.2. Pharmacodynamics
Effect of KISUNLA on Amyloid Beta Pathology
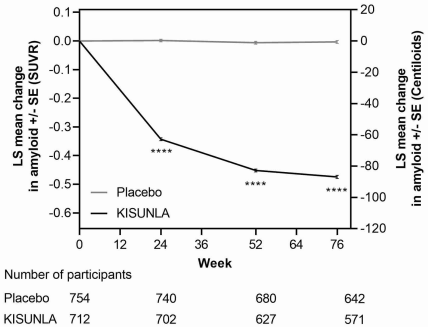
The effect of KISUNLA on amyloid beta plaque levels in the brain was evaluated using amyloid Positron Emission Tomography (PET) imaging (18F-florbetapir tracer). The PET signal was quantified using the Standard Uptake Value Ratio (SUVR) method to estimate brain levels of amyloid beta plaque in composites of brain areas expected to be widely affected by Alzheimer’s disease pathology (precuneus, frontal, anterior cingulate, posterior cingulate, parietal, and temporal cortices), compared to a brain region expected to be spared of such pathology (cerebellum). Results of amyloid PET were also expressed on the Centiloid scale.
In Study 1 [see Clinical Studies (14)], KISUNLA reduced amyloid beta plaque levels in the brain in a time-dependent manner, starting at Week 24, and continuing through Week 76 (p<0.0001), compared to placebo (see Figure 1 and Table 7). In clinical pharmacology studies, KISUNLA demonstrated a dose- and time-dependent reduction in amyloid beta plaque, with the decrease observed starting at Week 12.
Figure 1. Reduction in Brain Amyloid Beta Plaque (Change from Baseline) on Amyloid Beta PET Imaging Composite (SUVR and Centiloids) in Study 1a:
a ****p<0.0001.
During an off-treatment period, amyloid PET values began to increase with a median rate of 2.80 Centiloids/year.
Effect of KISUNLA on Tau Pathophysiology
A reduction in plasma p-tau217 was observed with KISUNLA compared to placebo in Study 1 (see Table 7).
Table 7. Biomarker Results of KISUNLA in Study 1 (AACI):
| Biomarker Endpoint at Week 76 | KISUNLA | Placebo |
|---|---|---|
| Amyloid Beta PET SUVR | N=712 | N=754 |
| Mean baseline | 1.53 | 1.52 |
| Adjusted mean change from baseline | -0.47 | -0.00 |
| Difference from placebo | -0.47, p<0.0001 | |
| Amyloid Beta PET Centiloid | N=765 | N=812 |
| Mean baseline | 104.0 | 101.8 |
| Adjusted mean change from baseline | -87.0 | -0.7 |
| Difference from placebo | -86.4, p<0.0001 | |
| Plasma p-tau217 (log10 transformed)a | N=758 | N=786 |
| Mean baseline | 0.67 | 0.66 |
| Adjusted mean change from baseline | -0.19 | 0.03 |
| Difference from placebo | -0.22, p<0.0001 |
N is the number of patients with baseline value.
a Results should be interpreted with caution due to the uncertainties in bioanalysis.
Exposure-Response Relationships
Model based exposure-response analyses for Study 1 demonstrated that exposures to donanemab-azbt were associated with a reduction in clinical decline on iADRS and CDR-SB. An association between reduction in amyloid beta plaque from baseline and clinical decline on iADRS and CDR-SB was also observed.
12.3. Pharmacokinetics
The pharmacokinetics (PK) of KISUNLA were characterized using a population PK analysis with concentration data collected from 2131 patients with Alzheimer’s disease who received KISUNLA in single or multiple doses. Accumulation of <1.3-fold occurs with every-4-week dosing; steady-state exposures are achieved after a single dose. In single doses from 10 to 40 mg/kg (~2 times the approved recommended dosage of 1400 mg for 70 kg of body weight), and multiple 10 and 20 mg/kg doses, exposures (Cmax and AUC) increased proportionally.
Distribution
The central volume of distribution is 3.36 L.
Elimination
KISUNLA is expected to be degraded by proteolytic enzymes in the same manner as endogenous IgG. The mean terminal half-life of donanemab-azbt is approximately 12.1 days. Donanemab-azbt clearance is 0.0255 L/h.
Specific Populations
Age, sex, or race were not found to affect the pharmacokinetics of donanemab-azbt. While body weight was found to influence both clearance and volume of distribution, the resulting changes were not clinically significant.
Patients with Renal or Hepatic Impairment
No clinical studies were conducted to evaluate the pharmacokinetics of donanemab-azbt in patients with renal or hepatic impairment. Donanemab-azbt is degraded by proteolytic enzymes and is not expected to undergo renal elimination or metabolism by hepatic enzymes.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies have not been conducted.
Mutagenesis
Genotoxicity studies have not been conducted.
Impairment of Fertility
No studies in animals have been conducted to assess the effects of donanemab-azbt on male or female fertility.
14. Clinical Studies
The efficacy of KISUNLA was evaluated in a double-blind, placebo-controlled, parallel-group study (Study 1, NCT04437511) in patients with Alzheimer’s disease (patients with confirmed presence of amyloid pathology and mild cognitive impairment or mild dementia stage of disease, consistent with Stage 3 and Stage 4 Alzheimer’s disease). Patients were enrolled with a Mini-Mental State Examination (MMSE) score of ≥20 and ≤28 and had a progressive change in memory function for at least 6 months. Patients were included in the study based on visual assessment of tau PET imaging with flortaucipir and standardized uptake value ratio (SUVR). Patients were enrolled with or without concomitant approved therapies (cholinesterase inhibitors and the N-methyl-D-aspartate antagonist memantine) for Alzheimer’s disease. Patients could enroll in an optional, long-term extension.
In Study 1, 1736 patients were randomized 1:1 to receive 700 mg of KISUNLA every 4 weeks for the first 3 doses, and then 1400 mg every 4 weeks (N=860) or placebo (N=876) for a total of up to 72 weeks. The treatment was switched to placebo based on amyloid PET levels measured at Week 24, Week 52, and Week 76. If the amyloid plaque level was <11 Centiloids on a single PET scan or 11 to <25 Centiloids on 2 consecutive PET scans, the patient was eligible to be switched to placebo.
Additionally, dose adjustments were allowed for treatment-emergent ARIA or symptoms that then showed ARIA-E or ARIA-H on MRI.
At baseline, mean age was 73 years, with a range of 59 to 86 years. Of the total number of patients randomized, 68% had low/medium tau level and 32% had high tau level; 71% were ApoE ε4 carriers and 29% were ApoE ε4 noncarriers. Fiftyseven percent of patients were female, 91% were White, 6% were Asian, 4% were Hispanic or Latino, and 2% were Black or African American.
The primary efficacy endpoint was change in the integrated Alzheimer’s Disease Rating Scale (iADRS) score from baseline to 76 weeks. The iADRS is a combination of two scores: the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog13) and the Alzheimer’s Disease Cooperative Study – instrumental Activities of Daily Living (ADCSiADL) scale. The total score ranges from 0 to 144, with lower scores reflecting worse cognitive and functional performance. Other efficacy endpoints included Clinical Dementia Rating Scale – Sum of Boxes (CDR-SB), ADAS-Cog13, and ADCS-iADL.
There were two primary analysis populations based on tau PET imaging with flortaucipir: 1) low/medium tau level population (defined by visual assessment and SUVR of ≥1.10 and ≤1.46), and 2) combined population of low/medium plus high tau (defined by visual assessment and SUVR >1.46) population.
Patients treated with KISUNLA demonstrated a statistically significant reduction in clinical decline on iADRS compared to placebo at Week 76 in the combined population (2.92, p<0.0001) and the low/medium tau population (3.25, p<0.0001).
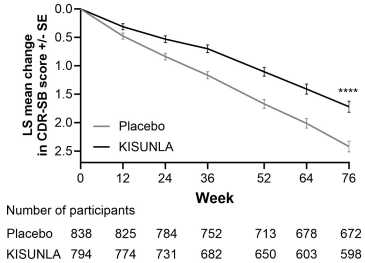
Patients treated with KISUNLA demonstrated a statistically significant reduction in clinical decline on CDR-SB compared to placebo at Week 76 in the combined population (-0.70, p<0.0001) (see Figure 2 and Table 8). There were also statistically significant differences (p<0.001) between treatment groups as measured by ADAS-Cog13 and ADCS-iADL at Week 76 (see Table 8).
Dosing was continued or stopped in response to observed effects on amyloid imaging. The percentages of patients eligible for switch to placebo based on amyloid PET levels at Week 24, Week 52, and Week 76 timepoints were 17%, 47%, and 69%, respectively. Amyloid PET values may increase after treatment with donanemab is stopped [see Clinical Pharmacology (12.2)]. There is no data beyond the 76-week duration of Study 1 to guide whether additional dosing with KISUNLA may be needed for longer-term clinical benefit.
Figure 2. CDR-SB Change From Baseline in Combined Population Through 76 Weeks in Study 1a:
a ****p<0.0001 versus placebo.
Table 8. Efficacy Analysis Results in Combined Population at Week 76a:
| Clinical Endpoints | KISUNLA (N=860) | Placebo (N=876) |
|---|---|---|
| CDR-SBb | ||
| Mean baseline | 3.92 | 3.89 |
| Adjusted mean change from baseline | 1.72 | 2.42 |
| Difference from placebo (%)d | -0.70 (29%) p<0.0001 | -- |
| ADAS-Cog13c | ||
| Mean baseline | 28.53 | 29.16 |
| Adjusted mean change from baseline | 5.46 | 6.79 |
| Difference from placebo (%)d | -1.33 (20%) p=0.0006 | -- |
| ADCS-iADLc | ||
| Mean baseline | 47.96 | 47.98 |
| Adjusted mean change from baseline | -4.42 | -6.13 |
| Difference from placebo (%)d | 1.70 (28%) p=0.0001 | -- |
a Abbreviations: ADAS-Cog13 = Alzheimer’s Disease Assessment Scale – 13-item Cognitive Subscale; ADCS-iADL = Alzheimer’s Disease Cooperative Study – instrumental Activities of Daily Living subscale; CDR-SB = Clinical Dementia Rating Scale – Sum of Boxes; NCS2 = natural cubic spline with 2 degrees of freedom; MMRM = mixed model for repeated measures.
b Assessed using MMRM analysis.
c Assessed using NCS2 analysis.
d Percent slowing of decline relative to placebo: difference of adjusted mean change from baseline between treatment groups divided by adjusted mean change from baseline of placebo group at Week 76.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.