KOSTAIVE Powder for dispersion for injection Ref.[114806] Active ingredients: Zapomeran
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Arcturus Therapeutics Europe B.V., Claude Debussylaan 10, 1082 MD Amsterdam, The Netherlands
4.1. Therapeutic indications
Kostaive is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in individuals 18 years of age and older.
The use of this vaccine should be in accordance with official recommendations.
4.2. Posology and method of administration
Posology
A single dose of 0.5 mL.
For individuals who have previously been vaccinated with a COVID-19 vaccine, Kostaive should be administered at least 5 months after the most recent dose.
Severely immunocompromised adults
Additional doses may be administered to individuals who are severely immunocompromised in accordance with official recommendations (see section 4.4).
Paediatric population
The safety and efficacy of Kostaive in children and adolescents less than 18 years of age have not been established. No data are available.
Elderly
No dose adjustment is required in elderly individuals ≥60 years of age.
Method of administration
Kostaive must be administered intramuscularly after reconstitution (see section 6.6).
Preferred site for the intramuscular injection is the deltoid muscle of the upper arm.
Use of a needle length appropriate for intramuscular injection is recommended.
The vaccine should not be injected intravascularly, subcutaneously, or intradermally.
The vaccine should not be mixed with any other vaccines or medicinal products in the same syringe.
For precautions to be taken before and after administering the vaccine, see section 4.4.
For instructions on reconstitution of the vaccine before administration, see section 6.6.
4.9. Overdose
In the event of an overdose, monitoring of vital functions and possible symptomatic treatment is recommended.
6.3. Shelf life
Unopened vial:
2 years at -15°C to -25°C.
Vials can be stored at room temperature (up to 25°C) for up to 4 hours before reconstitution.
Reconstituted medicinal product:
After preparation, the reconstituted vaccine vial must be stored under refrigerated conditions or at room temperature (2°C to 25°C) prior to administration and must be administered within 6 hours of initial puncture of the vial stopper.
Once thawed or reconstituted, the vaccine should not be refrozen.
Chemical and physical in-use stability has been demonstrated for 6 hours at 2°C to 25°C and includes transportation during this time. From a microbiological point of view, once the vial stopper has been punctured for reconstitution of the vaccine, the product may be stored for a maximum of 6 hours under refrigerated conditions or at room temperature (2°C to 25°C). Other in-use storage times and conditions are the responsibility of the user.
The reconstituted vaccine should be disposed of after 6 hours.
6.4. Special precautions for storage
Store in a freezer at -15°C to -25°C. Store in the original carton in order to protect from light.
For storage conditions after thawing and reconstitution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Powder contained in a vial (type I glass) with a stopper (bromobutyl rubber) and a plastic flip-off cap with seal (aluminum crimp).
Each multidose vial contains 16 doses of 0.5 mL; see section 6.6.
Pack size: 20 multidose vials.
6.6. Special precautions for disposal and other handling
Handling instructions
The vaccine should be prepared by a healthcare professional using aseptic techniques to ensure the sterility of the prepared dispersion.
ONLY use 10 mL of sterile sodium chloride 9 mg/mL (0.9%) solution for injection or equivalent for reconstitution.
Upon reconstitution, the vaccine is a white to off-white opalescent suspension (pH: 7.5-8.5); osmolality: 300-400 mOsm/kg.
After reconstitution, each vial contains 16 doses of 0.5 mL.
Extract 0.5 mL of vaccine into individual use syringes.
- Each dose must contain 0.5 mL of reconstituted dispersion for injection.
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.5 mL, do not administer the remainder. Instead, discard the vial and any remaining volume.
- Excess vaccine from multiple vials should not be pooled.
- After preparation, the filled syringes must be stored under refrigerated conditions or at room temperature (2°C to 25°C) prior to administration (including transportation during this time) and must be administered within 6 hours of initial puncture of the vial stopper.
- The reconstituted vaccine should be disposed of after 6 hours.
| Preparation of individual doses of Kostaive powder for dispersion for injection | |
| STEP A. Visual inspection and temperature equilibration of vials 1. Bring the vial to room temperature for at least one hour. Vials can be stored at room temperature (up to 25°C) for up to 4 hours before reconstitution. 2. Visually inspect for discolouration and gross defects/damages to container closure (e.g., breaks, glass shards, loose caps, missing stoppers, etc.). • Vial should contain white/off-white solid. DO NOT USE if there is damage to the container or other defects. DO NOT USE if unpunctured vial is at room temperature longer than 4 hours. | |
| STEP B. Addition of saline to vaccine 1. Reconstitution should be done immediately following complete temperature equilibration. 2. Obtain sodium chloride 9 mg/mL (0.9%) solution for injection (saline). Using a new sterile 10-mL syringe and 23G needle, withdraw 10 mL of sodium chloride 9 mg/mL (0.9%) solution for injection. 3. Remove the vial flip-off cap. 4. Use an alcohol wipe on the vial stopper. To ensure that 10 mL of sodium chloride 9 mg/mL (0.9%) solution for injection is added, the syringe should not be removed from the vial during steps 5-8. 5. Puncture the stopper with the saline syringe needle. • Record the date and time of initial stopper puncture and the time at which the vaccine should be discarded. (Note that vaccine must be administered within 6 hours of this stopper puncture.) 6. Slowly add half (5 mL) of the 10 mL of sodium chloride 9 mg/mL (0.9%) solution for injection into the vial along the sidewall. 7. Equalise the vial pressure by withdrawing approximately 3 mL of air from the vial into the saline syringe while keeping the needle above the liquid. 8. For the second and third additions of sodium chloride 9 mg/mL (0.9%) solution for injection, add 2 to 3 mL, directing the solution flow onto the inside wall of the product vial. • Follow each addition with a withdrawal of air from the vial using the saline syringe to equalise the vial pressure. Repeat the steps as needed to complete the addition of all 10 mL of sodium chloride 9 mg/mL (0.9%) solution for injection. Do not add more than 10 mL of sodium chloride 9 mg/mL (0.9%) solution for injection. | 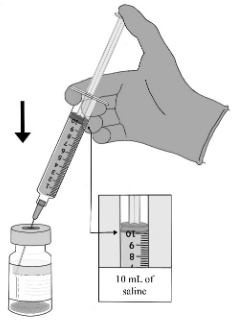 |
| STEP C. Equalise vial pressure 1. After the addition of sodium chloride 9 mg/mL (0.9%) solution for injection is complete, equalise the pressure before removing the needle from the vial by withdrawing air to the 8-mL line of the empty saline syringe. 2. Take care to adjust the position of the needle to be above the solution (to avoid inadvertent withdrawal of the reconstituted vaccine). 3. Remove the empty saline syringe and needle from the vial and discard. | 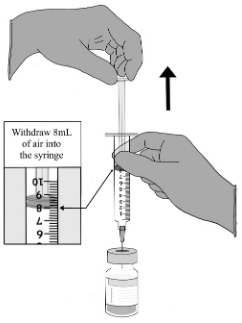 |
| STEP D. Mix and visually inspect the reconstituted vaccine 1. Gently invert the vial repeatedly for at least 1 minute until the solid is completely reconstituted. • Do not shake or vortex. • Avoid foaming and frothing. 2. Visually inspect the vaccine vial for particulates and discolouration. The liquid should be a white to off-white opalescent suspension. • DO NOT USE if particulates or discolouration are observed. 3. The reconstituted vaccine vial or filled syringes must be stored under refrigerated conditions or at room temperature (2°C to 25°C) prior to administration and must be used within 6 hours of initial stopper puncture. | 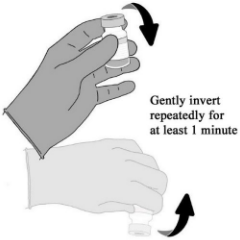 |
| STEP E. Preparation of syringe 1. Ensuring that there are no air bubbles, withdraw 0.5 mL of the vaccine using a sterile 1 mL syringe. 2. Record the date and time of initial stopper puncture. • Each filled syringe will be used for one dose. • Store filled syringes under refrigerated conditions or at room temperature (2°C to 25°C) prior to administration. • Each syringe should be used as soon as possible but must be used within 6 hours of the initial stopper puncture of the vial. | 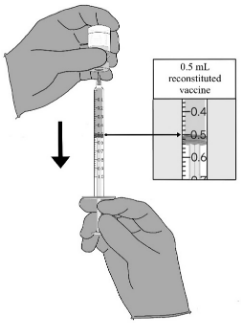 |
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.